Night work and cancer onset.
Catti Veronica, Prunelli Alberto
A critical and underrated aspect of sleeping alteration concerns the possible risk of cancer. At the base of this there would be a disruption of normal circadian rhythms.
Peaple with night working jobs may have altered levels of nocturnal melatonin and altered profiles of reproductive hormones, which could increase the risk of hormone-related diseases, including breast cancer.
Increasing evidence from epidemiological studies indicate that women who work at night, which have a prolonged sleep deprivation, abnormal circadian rhythms and light exposure at night have an increased risk of breast cancer, and probably also of colon and endometrium cancer (in man prostatic cancer). In addition there would also their more rapid progression.
Because shift work and night work are on the increase in Western societies, this exposure to increasing changes in the sleep-wake cycle and circadian rhythms associated is of the utmost importance from the point of view of social security, also because this aspect may explain a major factor in the increased cancers, especially breast, in the high-income world.
Circadian Disruption, Shift Work and the Risk of Cancer: A Summary of the Evidence and Studies in Seattle
Scott Davis, Dana K. Mirick 2006
Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report
Richard G Stevens 2011
Circadian rhythm and melatonin
A circadian rhythm is any biological process that displays an endogenous, entrainable oscillation of about 24 hours. These rhythms are driven by a circadian clock, and rhythms have been widely observed in plants, animals, fungi and cyanobacteria.
The term circadian comes from the Latin circa, meaning "around" (or "approximately"), and diem or dies, meaning "day".
Although circadian rhythms are endogenous ("built-in", self-sustained), they are adjusted (entrained) to the local environment by external cues called zeitgebers, commonly the most important of which is day-light.
In humans, the pineal gland lies in the center of the brain, behind the third ventricle.
The gland consists of two types of cells: pinealocytes, which predominate and produce both indolamines (mostly melatonin) and peptides (such as arginine vasotocin), and neuroglial cells. The gland is highly vascular.
In the biosynthesis of melatonin, tryptophan is first converted by tryptophan hydroxylase to 5-hydroxytryptophan, which is decarboxylated to serotonin. The synthesis of melatonin from serotonin is catalyzed by two enzymes (arylalkylamine N-acetyltransferase and hydroxyindole-O -methyltransferase) that are largely confined to the pineal gland.
The mammalian pineal gland is a neuroendocrine transducer. Photic information from the retina is transmitted to the pineal gland through the suprachiasmatic nucleus of the hypothalamus and the sympathetic nervous system. The neural input to the gland is norepinephrine, and the output is melatonin.
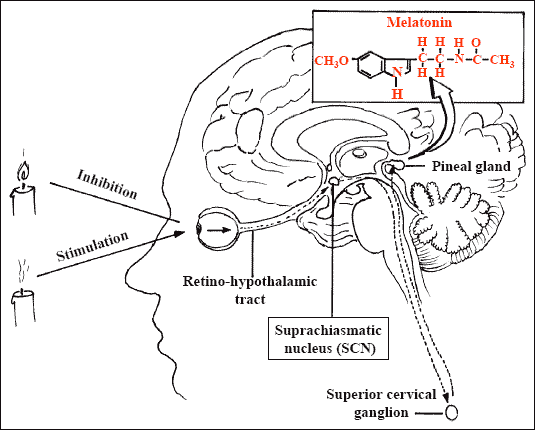
The synthesis and release of melatonin are stimulated by darkness and inhibited by light. During daylight hours, the retinal photoreceptor cells are hyperpolarized, which inhibits the release of norepinephrine. The retinohypothalamic–pineal system is quiescent, and little melatonin is secreted. With the onset of darkness, the photoreceptors release norepinephrine, thereby activating the system, and the number of α1- and β1-adrenergic receptors in the gland increases. The activity of arylalkylamine N-acetyltransferase, the enzyme that regulates the rate of melatonin synthesis, is increased, initiating the synthesis and release of melatonin.

In humans, melatonin secretion increases soon after the onset of darkness, peaks in the middle of the night (between 2 and 4 a.m.), and gradually falls during the second half of the night. Serum melatonin concentrations vary considerably according to age. The daytime rhythm in serum melatonin concentrations parallels the day–night cycle.However, a rhythm of about 24 hours' duration also persists in normal subjects kept in continuous darkness.
The circadian rhythm of melatonin secretion is of endogenous origin, reflecting signals originating in the suprachiasmatic nucleus. Environmental lighting does not cause the rhythm but entrains it (alters its timing). Light has two effects on melatonin: day–night light cycles modify the rhythm of its secretion.

Serum Melatonin Concentrations in Four Normal Men (22 to 35 Years Old) Living under Normal Light Conditions (Solid Circles) and after Living under Reversed Light Conditions for Seven Days and Six Nights (Open Circles).
Brief pulses of light of sufficient intensity and duration abruptly suppress its production. In normal subjects, exposure to light inhibits melatonin secretion in a dose-dependent manner. The threshold is 200 to 400 lux (equivalent to ordinary fluorescent light), and maximal inhibition occurs after exposure to intense light (600 lux or higher) for one hour. A longer exposure to light has no further suppressive effect on serum melatonin concentrations. Some blind persons with no pupillary light reflexes and no conscious visual perception have light-induced suppression of melatonin secretion, suggesting the existence of two photoreceptive systems: one mediating melatonin secretion and the other mediating the conscious perception of light.
Mechanisms of action: receptors
Two membrane-bound melatonin-binding sites belonging to pharmacologically and kinetically distinct groups have been identified: ML1 (high-affinity [picomolar]) sites and ML2 (low-affinity [nanomolar]) sites. Activation of ML1 melatonin receptors, which belong to the family of guanosine triphosphate–binding proteins (G protein–coupled receptors), results in the inhibition of adenylate cyclase activity in target cells. These receptors are probably involved in the regulation of retinal function, circadian rhythms, and reproduction. The ML2 receptors are coupled to the stimulation of phosphoinositide hydrolysis, but their distribution has not been determined.

Melatonin may also act at intracellular sites. Through binding to cytosolic calmodulin, the hormone may directly affect calcium signaling by interacting with target enzymes such as adenylate cyclase and phosphodiesterase, as well as with structural proteins. Melatonin has recently been identified as a ligand for two orphan receptors (α and β) in the family of nuclear retinoid Z receptors. The binding was in the low nanomolar range, suggesting that these receptors may be involved in nuclear signaling by the hormone.
Autoradiography and radioreceptor assays have demonstrated the presence of melatonin receptors in various regions of the human brain and in the gut, ovaries, and blood vessels. Neural receptors (e.g., those in the suprachiasmatic nucleus of the hypothalamus) are likely to regulate circadian rhythms. Non-neural melatonin receptors (such as those located in the pars tuberalis of the pituitary) probably regulate reproductive function, especially in seasonally breeding species, and receptors located in peripheral tissues (e.g., arteries) may be involved in the regulation of cardiovascular function and body temperature.
Melatonin is rapidly metabolized, chiefly in the liver, by hydroxylation (to 6-hydroxymelatonin) and, after conjugation with sulfuric or glucuronic acid, is excreted in the urine. The urinary excretion of 6-sulfatoxymelatonin (the chief metabolite of melatonin) closely parallels serum melatonin concentrations.
Enhancement of Immune Function
Melatonin may exert certain biologic effects (such as the inhibition of tumor growth and counteraction of stress-induced immunodepression) by augmenting the immune response. Studies in mice have shown that melatonin stimulates the production of interleukin-4 in bone marrow T-helper cells and of granulocyte–macrophage colony-stimulating factor in stromal cells, as well as protecting bone marrow cells from apoptosis induced by cytotoxic compounds. The purported effect of melatonin on the immune system is supported by the finding of high-affinity (Kd, 0.27 nM) melatonin receptors in human T lymphocytes (CD4 cells) but not in B lymphocytes.
Alteration of circadian rhythm
In humans, the circadian rhythm for the release of melatonin from the pineal gland is closely synchronized with the habitual hours of sleep. Alterations in synchronization due to phase shifts (resulting from transmeridian airline flights across time zones or unusual working hours) or blindness are correlated with sleep disturbances. In the initial description of melatonin as a melanophore-lightening agent, its sedative effect in humans was noted. More recently, serum melatonin concentrations were found to be significantly lower, with later peak nighttime concentrations, in elderly subjects with insomnia than in age-matched controls without insomnia. Electrophysiologic recordings demonstrated that the timing of the steepest increase in nocturnal sleepiness (the “sleep gate”) was significantly correlated with the rise in urinary 6-sulfatoxymelatonin excretion.
…and the insorgence of CANCER
There is evidence from experimental studies that melatonin influences the growth of spontaneous and induced tumors in animals. Pinealectomy enhances tumor growth, and the administration of melatonin reverses this effect or inhibits tumorigenesis caused by carcinogens.
Low serum melatonin concentrations and low urinary excretion of melatonin metabolites have been reported in women with estrogen-receptor–positive breast cancer and men with prostatic cancer. In addiction to this higher melatonin levels, as measured in first morning urine, are associated with a lower risk of breast cancer.
( Urinary melatonin levels and breast cancer risk. Schernhammer ES, Hankinson SE 2009
The mechanism by which melatonin may inhibit tumor growth is not known.
One possibility is that the hormone has antimitotic activity. Physiologic and pharmacologic concentrations of melatonin inhibit the proliferation of cultured epithelial breast-cancer cell lines (particularly MCF-7) and malignant-melanoma cell lines (M-6) in a dose-dependent manner. This effect may be the result of intranuclear down-regulation of gene expression or inhibition of the release and activity of stimulatory growth factors.
Melatonin may also modulate the activity of various receptors in tumor cells. For example, it significantly decreased both estrogen-binding activity and the expression of estrogen receptors in a dose-specific and time-dependent manner in MCF-7 breast-cancer cells.
Another possibility is that melatonin has immunomodulatory activity. In studies in animals, melatonin enhanced the immune response by increasing the production of cytokines derived from T-helper cells (interleukin-2 and interleukin-4), and as noted earlier, in mice melatonin protects bone marrow cells from apoptosis by enhancing the production of colony-stimulating factor by granulocytes and macrophages.
Lastly, as a potent free-radical scavenger, melatonin may provide protection against tumor growth by shielding molecules, especially DNA, from oxidative damage.
Night work and breast cancer risk: A systematic review and meta-analysis
Sarah P. Megdala 2005
Treatment with melatonin
Melatonin, when taken as a supplement, can stop or slow the spread of cancer, make the immune system stronger, or slow down the aging process.
The effects of melatonin have been studied in some patients with cancer, most of whom had advanced disease. In these studies, melatonin was generally given in large doses (20 to 40 mg per day orally) in combination with radiotherapy or chemotherapy. In a study of 30 patients with glioblastomas, the 16 patients treated with melatonin and radiotherapy lived longer than the 14 patients treated with radiation alone. In another study by the same investigators, the addition of melatonin to tamoxifen in the treatment of 14 women with metastatic breast cancer appeared to slow the progression of the disease. In a study of 40 patients with advanced malignant melanoma treated with high doses of melatonin (up to 700 mg per day), 6 had transient decreases in the size of some tumor masses. It has been claimed that the addition of melatonin to chemotherapy or radiotherapy attenuates the damage to blood cells and thus makes the treatment more tolerable. All these preliminary results must be confirmed in much larger groups followed for longer periods of time.
Light-at-Night-Induced Circadian Disruption, Cancer and Aging. Anisimov 2012
Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. P. Lissoni