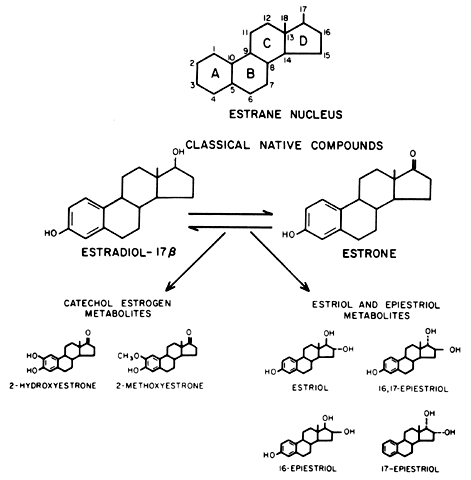
Cathecol Estrogens

https://media.springernature.com/lw685/springer-static/image/art%3A10.1186%2Fs40169-016-0088-3/MediaObjects/40169_2016_88_Fig2_HTML.gif?as=webp
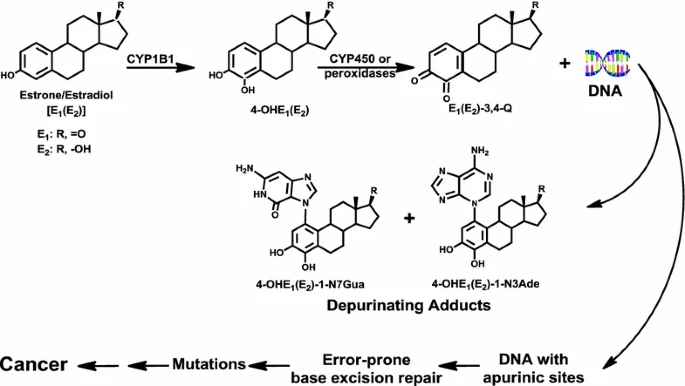
Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention 2016
Fig
Effects of exogenous thyroxine on C-2 and C-16 alpha hydroxylations of estradiol in humans. 1990
- Thyroid dysfunction in humans is known to alter the excretory pattern of estrogen metabolites, suggesting that thyroid hormone directly influences the oxidative metabolism of estradiol. We examined the extent to which a brief period of hyperthyroidism specifically affected estradiol hydroxylation at C-2 and C-16 alpha, the two primary and competing sites of estrogen oxidation, using an in vivo radiometric assay in healthy male volunteers. Hydroxylation at C-2 was increased by a 2-week course of thyroxine (4.3 micrograms/kg/d) from 29.9% +/- 2.6% to 35.9% +/- 3.1% (P = 0.04), while 16 alpha-hydroxylation was unchanged (10.3% +/- 0.8% versus 9.3% +/- 0.5%). The greater extent of oxidation at C-2 was evidenced by a twofold increase in the urinary excretion of 2-hydroxyestrone (2.88 +/- 0.32 versus 5.30 +/- 0.85 micrograms/g creatinine), while the excreted products of 16 alpha-hydroxylation were unchanged. At the same time, significant reductions in total cholesterol (173.8 +/- 7.9 versus 139.4 +/- 8.9 mg/dl), low-density lipoprotein cholesterol (110.0 +/- 5.3 versus 83.8 +/- 7.7 mg/dl), and apolipoprotein B (68.2 +/- 3.3 versus 53.1 +/- 3.6 mg/dl) were observed. Serum levels of estrone, estradiol, sex hormone-binding globulin, high-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, triglycerides, and apolipoprotein A-I were not significantly affected. This study adds to the growing evidence that catechol estrogen production in humans is more readily regulated than 16 alpha-hydroxylation, which is relatively refractory to treatment.

C2 and C4 hydroxylation
cyp?
17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. 1996
The 4-hydroxy metabolite of 17 beta-estradiol (E2) has been implicated in the carcinogenicity of this hormone.
Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney 1994
The coadministration of estradiol plus 0.3 or 3% quercetin for 16 days increased renal NADPH-dependent estradiol-4- but not estradiol-2-hydroxylase activities by 91 or 73%, respectively, over values obtained with hormone treatment alone.
Opiate regulation of estradiol-2-hydroxylase in brains of male rats: mechanism for control of pituitary hormone secretion. 1980
The injection of naloxone (0.4 mg/kg) alone produces a significant increase in brain estradiol-2-hydroxylase activity over control levels.
The oxidative metabolism of estradiol: inhibition by cimetidine. 1989
The inhibition of estradiol 2-hydroxylation and the increase in serum estradiol concentrations caused by cimetidine administration may help to account for the symptoms of hyperestrogenization reported in long-term cimetidine therapy.
ovarian steroidogenesis
CYP4501A1 in 2
CYP4501B1 in 4
Downregulation of the male-specific hepatic microsomal steroid 16 alpha-hydroxylase, cytochrome P-450UT-A, in rats with portal bypass. Relevance to estradiol accumulation and impaired drug metabolism in hepatic cirrhosis. 1989
The decreased activity of hepatic estradiol 16 alpha-hydroxylation after PVL would enhance the accumulation of estradiol, the biologically more potent estrogen.
COMT activity

Estriol cathecol?
Breast cancer risk and methylenetetrahydrofolate reductase polymorphism. 2003
- OBJECTIVE Methylenetetrahydrofolate reductase (MTHFR), a polymorphic enzyme involved in folate metabolism, plays a role in DNA biosynthesis, methylation, and repair in actively dividing cells. Because breast-cell division occurs in women with active ovulatory cycles, polymorphisms in the MTHFR gene could be a risk factor for breast cancer.
METHODS We genotyped 352 clinic-based study subjects for MTHFR, 105 subjects with breast cancer and 247 with benign breast disease, histopathologically classified as high-risk or low-risk for breast cancer. Questionnaire data were collected prior to biopsy to blind subjects and interviewers to diagnoses.
RESULTS Premenopausal women with the MTHFR polymorphism had a threefold increased breast cancer risk (OR = 2.8; 95%CI: 1.02-7.51) compared to the clinic-based controls with benign breast disease. Results were similar using either low- or high-risk controls. However, risk for postmenopausal women was not elevated (OR = 0.8; 95%CI 0.4-1.4). No significant interaction between genotype and smoking or alcohol was found, but polymorphic MTHFR decreased the likelihood of drinking alcohol (OR = 0.5; 95%CI 0.3-0.9).
CONCLUSION These data suggest that polymorphic MTHFR increases risk of premenopausal, but not postmenopausal, breast cancer. These findings should be explored with a larger sample size in order to analyze gene-environment interactions between MTHFR and folate. Once the intricate relationship between diet and breast cancer has been elucidated, new cancer control initiatives can be considered such as folate chemoprevention trials in high-risk individuals.