DIPHTHERIA and ADP-ribosyl transferase effects
Diphtheria (Greek διφθέρα (diphthera) "pair of leather scrolls") is an upper respiratory tract illness caused by Corynebacterium diphtheriae (from 0.3-0.8 × 1.0 to 8.0 micron) , aerobic/facultative anaerobic, Gram-positive, static and asporigenic bacterium with a cudgel form and a disposition that simulates 'chinese letters'. C. Diphtheriae was discovered by Edwin Klebs in 1883.

Pathology
Pathology is characterized by sore throat, low fever and adherent membranes ( pseudomembranes ) on the tonsils, pharynx and/or nasal cavity. These pseudomembranes are formed by bacteria, necrotic epithelial cells, neutrophil granulocytes and fibrin. They can grow and extend to soft palate, hard palate, larynx and trachea; pseudomembranes are important in mechanic damage, because they can occlude respiratory tract ( croup ). The patient can die of suffocation because of pseudomembranes detachment that can involve in the larynx obstruction. A milder form of diphtheria can be restricted to the skin, expecially in tropical areas; it can lead to a gangrene. Less common consequences include myocarditis (about 20% of cases), cardiac arrhythmias and cranial/peripheral neuropathy (about 10% of cases).
Diphtheria is a contagious disease spread by direct physical contact or breathing the aerosolized secretions of infected individuals.
The pathology of diphtheria. (February, 2000)

Signs and symptoms
Incubation period lasts for two to seven days; then it appears with fever of 38°C or above, chills, fatigue, bluish skin coloration, sore throat, hoarseness, cough, headache, difficulty swallowing (dysphagia), painful swallowing (odynophagia), lymphadenopathy, dysphonia and sometimes aphonia, rhinits, difficulty breathing, rapid breathing, foul-smelling bloodstained nasal discharge, oedma, erythema and kidney insufficiency.

Diphteria pantropic exotoxin
The most important pathology linked to Diphtheria is caused by a pantropic exotoxin produced during exponential increasing phase by C. diphtheriae only when infected with a β-bacteriophage that integrates the toxin-encoding genetic elements ( TOX gene ) into the bacteria. The gene regolation is up to the bacterium; there are also methabolic elements that contribute to the production regolation, for example iron intracellular content, that at high concentration activates diphtheria toxin repressor ( DTxR ) and consequently an interruption of toxin production [1]; otherwise if iron decreases there is a TOX gene derepression. The pantropic exotoxin affects many different cells that present specific receptors on their cell membrane: miocardium, spleen, kidneys, adrenal glands, periferic nerves. In order to identify Corynebacterium Diphtheriae productors of this exotoxin (TOX gene integration) it can be used a rapid enzyme immunoassay ( EIA ) or the Elek Test or PCR.
Rapid enzyme immunoassay for determination of toxigenicity among clinical isolates of corynebacteria. (April, 2000)
Exotoxic structure and mechanism
Diphtheria toxin is a single polypeptide chain of 565 amino acids consisting of two subunits linked by disulfide bridges, known as an A-B toxin. Binding to the cell surface of the B subunit allows the A subunit to penetrate the host cell.
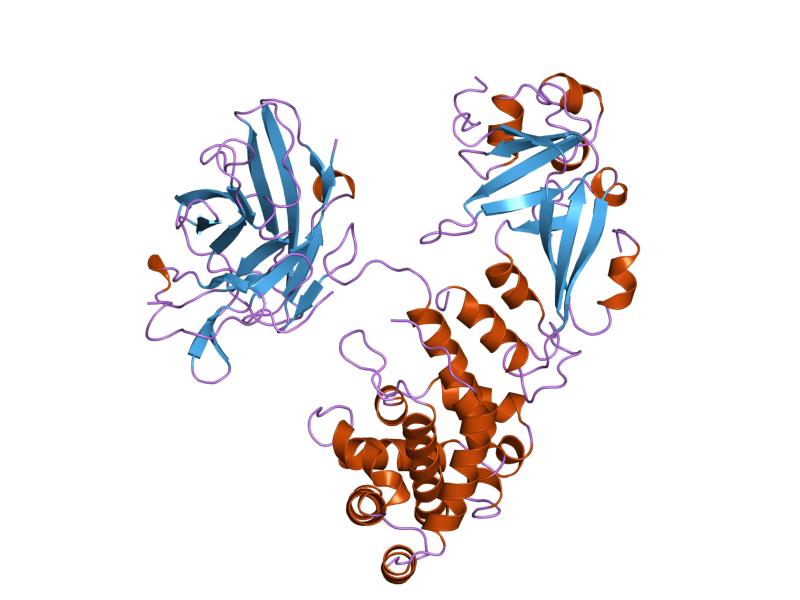
The crystal structure of the diphtheria toxin homodimer has been determined to 2.5 resolution. The structure reveals a Y-shaped molecule consisting of 3 domains. Fragment A contains the catalytic C domain, and fragment B consists of the T and R domains.
The N-terminal catalytic domain, known as the C domain, has an unusual beta+alpha fold. The C domain blocks protein synthesis in the affected cell by transfer of ADP-ribose from NAD to a unusual amino acid diphthamide a unique post-translationally modified histidine residue of EF-2, eukaryotic elongation factor-2 (EF-2 catalyze the translocation of the tRNA and mRNA down the ribosome at the end of each round of polypeptide elongation), inactivating this protein. In this way, it acts as a RNA translational inhibitor.
Diphthamide biosynthesis requires an organic radical generated by an iron–sulphur enzyme (June 17, 2010)

The cholera toxin and exotoxin A of Pseudomonas aeruginosa uses a similar mechanism of action.
A central translocation domain, known as the T domain or TM domain. The T domain has a multi-helical globin-like fold with two additional helices at N-termini, but which has no counterpart to the first globin helix. This domain is thought to unfold in the membrane. pH-induced conformational change in the T domain triggers insertion into the endosomal membrane and facilitates the transfer of the C domain into the cytoplasm.
A C-terminal receptor-binding domain, known as the R domain. This domain has a beta-sandwich fold consisting of nine strands in two sheets with greek-key topology; it is a subclass of immunoglobin-like fold. The R domain binds to cell surface receptor, permitting the toxin to enter the cell by receptor mediated endocytosis. The receptor is the epidermal growing factor which usually binds heparin, it is located on the surface of a lot of eucaryotic cells, in particular on cardiac and nervous ones. [2]
Aminoacid content of Diphtheria toxin per cent


Diphtheria toxin - P00588 (October 4, 2004)
Advantage for C. diphtheriae carried by the exotoxin
The invasion of a host by Corynebacterium may be aided by the production of bacterial extracellular substance (diphtheria exotoxin) which act against the host by breaking down primary or secondary defenses of the body. Diphtheria exotoxin is a protein (enzyme) that act locally to damage host cells and/or have the immediate effect of facilitating the growth and spread of the pathogen. The damage to the host as a result of this invasive activity may become part of the pathology of an infectious disease.
Exotoxin produced by bacteria promote their invasion and also damage the host. It is cytotoxic and may act at remote sites (removed from the site of bacterial growth). Also, exotoxin typically is more specific and more potent in their activity than invasins, proteins which usually act at a short range (in the immediate vicinity of bacterial growth) and may not actually kill cells as part of their range of activity. Exotoxin may play some role in colonization or invasion in the early stages of an infection.
The production of a toxin may play a role in adapting Corynebacterium to a particular niche, but it is not essential to the viability of the organism.
The Mechanisms of Bacterial Pathogenicity (2009)
Diagnosis
The main procedure to identify Corynebacterium diphtheriae is microscopic observation of pathologic material taken with a pharyngeal plug. In the same time it's carried out a cultural test to show methaphosphate methacromatic particles contained in Corynebacterium and red coloured if painted with the Methylene blue. For this test laboratorists use Löffler ground (coagulate serum) or Tinsdale ground (which contains K2TeO3).
Epidemiology
Diphtheria is a serious disease, with fatality rates between 5% and 10%. In children under five years and adults over 40 years, the fatality rate may be as much as 20%. It's endemic in developing countries such as Brasil, Nigeria, India and Indonesia and also in Russia (in the late 1980s), because of a suspension of massive vaccination, but outbreaks, though very rare, still occur worldwide, even in developed nations, such as Germany and Canada. This illness has a seasonal trend with its peak in winter months.
Therapy
Corynebacterium diphtheriae is sensible to penicillin, tetracycline, cephalosporin, metronidazole and erythromycin, but in case of C. Diphtheriae exotoxin productor antibiotic therapy is not effective because it has no effect on the toxin; on the other hand prophylaxis is important.
Prophylaxis
Diphtheria vaccine is a component of the DPT vaccine, a class of combination vaccines against three infectious diseases in humans: diphtheria, pertussis (whooping cough) and tetanus. The vaccine components include diphtheria and tetanus toxoids, and killed whole cells of the organism that causes pertussis. In case of contact with an affected person it can be useful do a passive immunization with antitoxin serums.
DTaP immunization (February 1, 2012)
Bibliography
- [1], pag. 246, Medical Microbiology, Patrick R. Murray, Michael A. Pfaller, Ken S. Rosenthal, 2009
- [2], pag. 246, Medical Microbiology, Patrick R. Murray, Michael A. Pfaller, Ken S. Rosenthal, 2009