DEFINITION
The phantom limb syndrome is the sensation that an amputated or missing limb is still attached to the body and is moving appropriately with other body parts. This is called phantom limb awareness, that is often accompanied by specific sensory kinaesthetic sensations (phantom sensations); patients sometimes feel as if they are gesturing, feel itches, twitch, or even try to pick things up.

Many individuals with amputations have a sensation that they are able to move their missing limbs voluntarily but others experience the missing limb as paralyzed in a painfully awkward position. Often, the patient feels to have a shorter limb than the other; this means that the exact sensation differs widely for individuals. Moreover, the pain can be made worse by stress, anxiety, and environmental changes (Rebecca Brightwell, AgrAbility in Georgia).
But the most important feature of this syndrome is the painful condition of the amputated limb, called Phantom Limb Pain (PLP).
SYMPTOMS
Phantom pains occur when nerves that would normally innervate the missing limb cause pain.
PLP has many features: at one end of the spectrum it is limited to simple, short-lasting and rarely occurring painful shocks in a missing body part; at the other end of the spectrum it can be a constant, excruciatingly painful experience during which the individual has a vivid and intense perception of the missing body part.
It seems to be more severe in the distal portions of the phantom and can have a number of characteristics such as cramping, shooting, stabbing or burning.
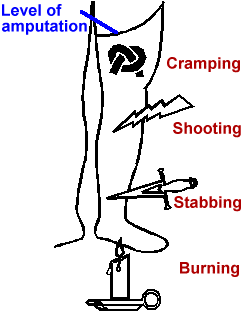
Its onset can be immediate, but it may also appear for the first time many years after the amputation.
Although phantom limb pain is more common after the amputation of an arm or leg, it may also occur after the surgical removal of other body parts, such as a breast, rectum, penis, testicles, eyes, tongue or teeth (Phantom pain after eye amputation, 2011; Phantom phenomena in mastectomized patients and their relation to chronic and acute pre-mastectomy pain, 2004).
Interestingly, phantom limb pain is more frequent when the amputation occurs in adulthood, less frequent in child amputees and virtually non existent in congenital amputees (Phantom limb sensations and phantom limb pain in child and adolescent amputees, 1998).
EPIDEMIOLOGY
Approximately 60 to 80% of individuals with an amputation experience phantom sensations in their amputated limb, and the majority of the sensations are painful.

Since the beginning of the fire conflicts in the world, there has been a dramatic increase in the number of military service members with single and multiple-limb amputations. Phantom limb pain (PLP) frequently develops in these individuals.
In Western countries, the main reason for amputation is chronic vascular disease. The patients are elderly and have often suffered from long-lasting pre-amputation been observed as a consequence of the loss of other pain.
In other parts of the world, civil wars and landmine explosions result in many unfortunate cases of traumatic amputations in otherwise healthy people (Phantom limb pain: theories and therapies, 2010).
RISK FACTORS
Recent studies report the prevalence of PLP to be more common among upper limb amputees than lower limb amputees; upper limb amputees are significantly more likely to suffer post-amputation pain which is more frequent, longer lasting and more severe in intensity when compared to lower limb amputees. Lower limb amputees fared better than upper limb amputees in terms of bodily pain, social function and mental health (A cross-sectional study of post-amputation pain in upper and lower limb amputees, experience of a tertiary referral amputee clinic, 2010).
Other risk factors are the presence of pre-amputation pain (Preamputation pain and acute pain predict chronic pain after lower extremity amputation, 2007) or even the time after amputation: phantom pain and phantom sensations are often long-term consequences of amputation. Amputees experience phantom sensations and phantom pain within 1 month after amputation, and a second peak occurs 12 months after amputation (Painful and nonpainful phantom and stump sensations in acute traumatic amputees, 2008).
It was also reported to be more common among females than males.

Female are hypothesized to report greater pain intensity and pain interference, and are also expected to report using a greater number of pain coping strategies and engaging in more frequent pain catastrophizing (Sex Differences in Pain and Psychological Functioning in Persons with Limb Loss, 2009).
Finally, the psychological aspect (such as stress, anxiety, depression) highly likely contribute to the persistence or exacerbation of PLP. A study has found that amputees with depressive symptoms were more likely to characterize their pain as more severe than those without depressive symptoms (Phantom pain, residual limb pain, and back pain in amputees: results of a national survey, 2005).
NEUROLOGICAL BASES
In a historical perspective, PLP had been considered mostly of psychological origin, with the prevalent belief that PLP was generated “in the patient’s head.” However, the development of advanced diagnostic methods, recently including neuroimaging, has facilitated explorations of changes in peripheral and central neural networks after amputation and their putative contribution to the development of PLP. The findings acknowledged the neuropathic nature of PLP and also suggested that both peripheral, as well as central mechanisms, including neuroplastic changes in central nervous system, can contribute to PLP.
PERIPHERAL CHANGES
Until recently, the dominant theory for cause of phantom limbs was irritation in the severed nerve endings (called neuromas). When a limb is amputated, many severed nerve endings are terminated at the residual limb.
As a consequence of injury, terminal swelling and regenerative sprouting of the injured axon end occurs and neuromas form in the residual limb that display spontaneous and abnormal evoked activity to mechanical and chemical stimuli. Ectopic discharges from stump neuromas represent a source of abnormal afferent input to the spinal cord and a potential mechanism for spontaneous pain and abnormal evoked pains.
The increased excitability of injured nerves that result in ectopic discharge seems to be due to alterations in the electrical properties of cellular membranes, such as an alteration of voltage-gated sodium channels and a decreasing of potassium channel expression in the neuroma (Sodium channels and mechanisms of neuropathic pain, 2006).

This is caused by an accumulation of molecules enhancing the expression of sodium channels in these neuromas, that results in hype-excitability and spontaneous discharges. Studies reporting the reduction of phantom pain with drugs blocking the sodium channels lend further support to this theory (The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome, 2010).
CENTRAL CHANGES
SPINAL CORD
Some neurons in the areas of spinal cord that are not responsible for pain transmission also sprout into the Lamina II of the dorsal horn of the spinal cord which is the area involved in the transmission of nociceptive afferent inputs. This is followed by increased neuronal activity (mechanical hyperalgesia) expansion of the neuronal receptive field, and hyperexcitability of other regions: this complicated process is called central sensitization.
Inhibitory GABA-containing and glycinergic interneurons in the spinal cord could be destroyed by rapid ectopic discharge, or might change from having an inhibitory to an excitatory effect under the influence of Brain-Derived Neurotrophic Factor (BDNF) released from the microglia, thereby contributing to a hyperexcitable spinal cord (BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain, 2005).

During this process, there is also an increase in the activity at NMDA receptors mediated by neurotransmitters such as substance P, tachykinins, and neurokinins at the dorsal horn of the spinal cord.
These factors are normally expressed only by C-afferents and Aδ afferents, most of which are nociceptors. But in the central sensitization, there is an injury-triggered expression of these factors (as Substance P) by Aβ fibres, that might contribute to Phantom Limb Pain.
This process brings about a complicated change in the firing pattern of the central nociceptive neurons (Phantom Limb Pain: Mechanisms and Treatment Approaches, 2011).

CORTICAL REORGANIZATION
Recent studies demonstrate some changes in the funcional and structural architecture of the primary somatosensory cortex (SI), subsequent to amputation; this is called cortical reorganization, and it's perhaps the most cited reason for the cause of PLP in recent years. During reorganization, the cortical areas representing the amputated extremity are taken over by the neighboring representational zones in the primary somatosensory cortex. The process and extent of cortical reorganization have been studied in both animal and human models following amputation and deafferentation. Cortical reorganization explains why the afferent nociceptive stimulation of neurons within the stump or surrounding area produces the sensation in the missing limb (Phantom limb pain: a case of maladaptive CNS plasticity?, 2006).

Ramachandran, one of the most famous scientists about this topic, showed a point-to-point correspondence between stimulation sites and areas of sensation from the face to the phantom in arm amputees.
This happens because the sensory input from face invade the original hand area in the brain, so the simple action of touching the face of the amputee evokes tactile sensations on the phantom (e.g. there is a "thumb in cheek" map of stimulation on the face; this is the reason why an amputee who shaves, stimulating his cheek, might have phantom limb pain or phantom hand sensations).
These sensory referrals from the face to phantom hand occur in a stable, topographically organized manner (Dynamic reorganization of referred sensations by movements of phantom limbs, 2010).

Multiple imaging studies using functional MRI (fMRI) have correlated greater extent of somatosensory cortex involvement with more intense phantom limb experience; activation in primary somatosensory and motor cortices is unaltered in amputees without pain and is similar to that in healthy controls. In the amputees with PLP, the cortical representation of the mouth extends into the region of the hand and arm.
Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery, 2008).

NEW STUDIES IN THE ACC
Very recently (december 2012), a study reveals the importance of the cortical plasticity in the Anterior Cingulate Cortex (ACC), a critical cortical area for pain sensation, including Phantom Limb Pain.
Nerve injuries or amputations trigger a series of plastic changes in pain-related cortical areas including the ACC. The main event is a Loss of Long Term Depression (LTD) within the ACC and between cortical areas, that contributes to enhanced excitability of pain-processing and feeling cortical neurons. Some of these cortical changes may not require persistent peripheral sensory inputs; thus will not respond to any medical treatment that targeted at lower subcortical levels. In addition to contribution to pain or phantom pain, such cortical plastic changes may also triggers a series of brain disorders such as emotional fear, anxiety, mood depression, and impairment of cognitive functions (Cortical Depression and Potentiation: Basic Mechanisms for Phantom Pain, 2012).
THERAPIES
A number of different therapies relying on different principles have been proposed for the management of Phantom Limb Pain; however, specific treatment guidelines are yet to evolve and most successful measures employ multidisciplinary approaches in the management of pain and in rehabilitation.
Treatment guidelines used for other neuropathic pain conditions are probably the best approximation for now, especially for the treatment of stump pain (Postamputation pain: studies on mechanisms, 2012)
PHARMACOLOGICAL TREATMENTS
PARACETAMOL AND NSAIDS
A cross sectional study found that Acetaminophen (or Paracetamol) and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) were the most common medications used in the treatment of PLP. The analgesic mechanism of acetaminophen is not clear but serotonergic and multiple other central nervous system pathways are likely to be involved. Acetaminophen has a little anti-inflammatory activity as compared to NSAIDs.
NSAIDs inhibit the enzymes needed for the synthesis of prostaglandin and decrease the nociception peripherally and centrally.
OPIOIDS

Opioids bind to the peripheral and central opioid receptors and provide analgesia without the loss of touch, proprioception, or consciousness. They may also diminish cortical reorganization and thus disrupt one of the proposed mechanisms of PLP. Randomized controlled trials have demonstrated the effectiveness of opioids (oxycodone, methadone, morphine, and levorphanol) for the treatment of neuropathic pain, including PLP (Methadone for phantom limb pain, 2002).
In particular, morphine has been shown to be very beneficial for the management of PLP, while methadone has received a differential treatment due to the additional concern about cardiac toxicity (The effects of methadone and its role in fatalities, 2004).
Comparative trials have also shown benefit with opioids when compared with tricyclic antidepressants, but the total amount of opioid required to achieve analgesia may be less when used together with these tricyclic antidepressants or anticonvulsants, which also have use in neuropathic pain modulation.
NON PHARMACOLOGICAL TREATMENTS
TENS
Transcutaneous Electrical Nerve Stimulation has been found to be helpful in PLP.
It's a therapy generally used for neuropathic pain.
Low-frequency and high-intensity TENS is thought to be more effective than other doses. There are some studies showing the effectiveness of TENS made on the contralateral limb versus ipsilateral (the phantom limb) to decrease PLP (Contralateral stimulation, using TENS, of phantom limb pain: two confirmatory cases, 2010).
TENS devices are portable, are easy to use, and have few side effects or contraindications.

MIRROR THERAPY
It's maybe one of the most interesting and important treatments for the Phantom Limb Pain. Mirror therapy was first reported by Ramachandran in 1996 and is suggested to help PLP by resolving the visual-proprioceptive dissociation in the brain. The patient watches the reflection of his intact limb moving in a mirror placed parasagittally between their arms or legs (for the upper limb it's called mirror box), while simultaneously moving the phantom limb (moving the stump) in a manner similar to what he is observing so that the virtual limb replaces the phantom limb. Most of the patients reported a decreasing of the Phantom Limb Pain.

This can be explained by the existence in the brain of mirror neurons (neurons that are active not only during the execution of the task itself but also during the observation of the task), which fire in this occasion. The presence of mirror neurons in the brain is also supported by the phenomenon of tactile sensation in the phantom limb elicited by touching the virtual image of the limb (i.e. touching the contralateral limb, reflected in the mirror).
A person without phantom limb pain and no amputations cannot feel these tactile sensations, because inputs from a non-mirror neuron block the mirror neuron, while a patient with an amputation does not have this non-mirror neuron system operating.
Since the activation of these mirror neurons modulates somatosensory inputs, their activation may block protopathic pain perception in the phantom limb, and it is expected to decrease pain by resolving conflict between motor intention, proprioception and visual system.
Mirror therapy is expected to be widely used for the treatment of phantom limb pain, because it's easy to use, even at patient's home (Mirror Therapy for Phantom Limb Pain, 2012).
This video shows a soldier using mirror therapy for his right leg that lost in Baghdad, during the war in Iraq, on December 17th 2007.