Arginine (abbreviated as Arg or R) is an α-amino acid. In mammals, arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual (eg. infants, aged people ??)

Arginine metabolism
Arginine fates:
- Ornithine
- NO synthesis
- Proteins synthesis
- creatine
- agmatine
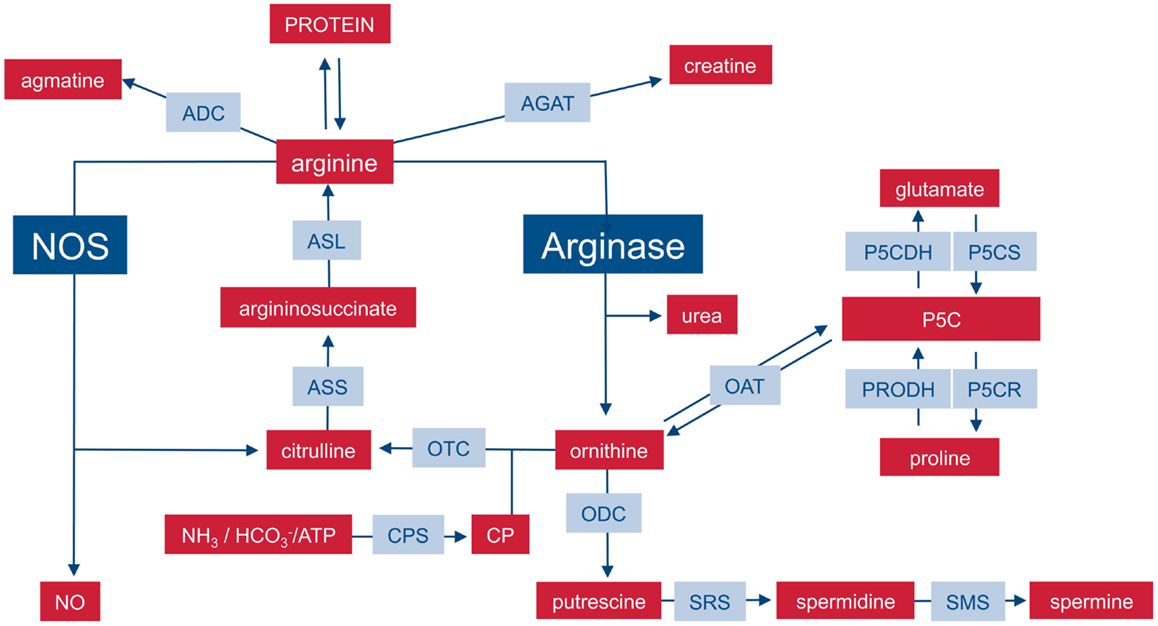
Medscape figure
Relative Rate of different Pathways
Arginine availability
- Diet
- Endogenous Synthesis
- Citrulline
- ASS (Argininosuccinate synthase: ATP + L-citrulline + L-aspartate = AMP + diphosphate + N(omega)-(L-arginino)succinate.)
- fumarate
- ASL (Argininosuccinate lyase: 2-(N(omega)-L-arginino)succinate = fumarate + L-arginine)
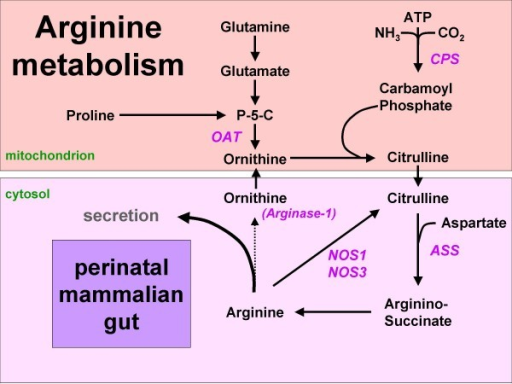
Figure 1: Arginine synthesis from proline or glutamine in the mammalian neonatal gut. Since arginase-1 is not expressed, arginine can either be secreted or metabolized to NO and citrulline. Names of enzymes investigated in this study are indicated in italics. P-5-C = pyrroline5-carboxylate synthetase.
The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and -catabolizing enzymes., 2008
Mentions: In the adult, endogenous arginine biosynthesis is an inter-organ 'affair': the net production of citrulline occurs almost exclusively in the enterocytes of the small intestine [1], also in man [2], but absorption of citrulline from the circulation and subsequent biosynthesis of arginine can take place in many tissues [3]. Of these, the cortex of the kidney provides approximately 20% of whole-body requirements [4]. In perinatal mice [5,6] and piglets [7-9], however, all enzymes necessary for arginine biosynthesis from proline and glutamine (Figure 1) are expressed in the enterocytes of the small intestine, while ARG1, the main cytosolic arginine-catabolizing enzyme, is not detectable prior to weaning [5,6,10]. In agreement, the small intestine plays a prominent role in net arginine production in suckling piglets [11-14]. In rodents, intestinal expression of the enzymes that synthesize arginine from citrulline, ASS and argininosuccinate lyase, ceases completely after weaning [6,15]. In pigs, on the other hand, net synthesis of arginine declines more gradually and is still present at 7 weeks of age [16]. It has been speculated that enteric arginine synthesis is necessary to cover neonatal requirements, because mammalian milk is a relatively poor source of arginine, whereas its precursors proline and glutamine are abundant [17].
Arginine from Urea cycle


Arginine and HSV
nutrient for HSV
Arginine and NO production
J Physiol. 2006 Jul 15;574(Pt 2):573-81. Epub 2006 May 4.
L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin.
Holowatz LA, Thompson CS, Kenney WL.
Department of Kinesiology, Noll Laboratory, Pennsylvania State University, University Park, PA 16802, USA. lma191@psu.edu
Full expression of reflex cutaneous vasodilatation is dependent on nitric oxide (NO) and vasodilatation is attenuated in healthy older humans. NO bioavailability in aged skin may be decreased by an age-related upregulation of arginase, which reciprocally regulates the NO-synthase (NOS) substrate L-arginine (L-Arg). We hypothesized that increased arginase activity contributes to attenuated vasodilatation in aged skin by limiting L-Arg for NOS-mediated NO synthesis. Five microdialysis fibres were placed in forearm skin of 10 young (Y, 23 +/- 1 years) and 9 older (O, 68 +/- 1 years) human subjects, serving as control (C, Ringer solution), NOS-inhibited (10.0 mM NG-nitro-L-arginine), arginase-inhibited (5.0 mM (S)-(2-boronoethyl)-L-cysteine + 5.0 mM Nomega-hydroxy-nor-L-arginine), L-arg supplemented (L-Arg; 10.0 mM L-arginine) and combined arginase-inhibited + L-Arg sites. After 20 min thermoneutral baseline, cutaneous vasodilatation was induced by passive whole-body heating to increase oral temperature (Tor) by 1.0 degrees C. Red blood cell flux was measured by laser-Doppler flowmetry over each microdialysis site. Cutaneous vascular conductance was calculated (CVC = flux/mean arterial pressure) and normalized to maximal CVC (CVCmax, 28.0 mM sodium nitroprusside + local heating to 43 degrees C). Cutaneous vasodilatation during heating was attenuated in O (Y, 42 +/- 1, versus O, 30 +/- 1%CVCmax, P < 0.001) at control sites. NOS inhibition decreased vasodilatation in both age groups compared to C (Y, 22 +/- 2; O, 18 +/- 2%CVCmax; P < 0.001). Arginase inhibition, L-Arg supplementation, and arginase inhibition + L-Arg supplementation augmented vasodilatation in O (arginase-inhibited, 46 +/- 4; L-Arg, 44 +/- 4; arginase-inhibited + L-arg, 46 +/- 5%CVCmax; P < 0.001 versus C) but not in Y (arginase-inhibited, 46 +/- 4; L-Arg, 38 +/- 4; arginase-inhibited + L-Arg, 44 +/- 4%CVCmax; P > 0.05 versus C). Increasing L-Arg for NO synthesis by either arginase inhibition or direct L-Arg supplementation restores the age-related deficit in reflex cutaneous vasodilatation.
Anti-inflammatory effect oral L-arginine supplementation
Regulation of immune responses by L-arginine metabolism

Metabolism. 2009 Sep;58(9):1270-6. Epub 2009 Jul 9.
Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass., 2009
Internal Medicine Department, Cardio-Diabetes Trials Unit, Scientific Institute San Raffaele, 20132 Milan, Italy. piatti.piermarco@hsr.it
It is known that L-arginine treatment can ameliorate endothelial dysfunction and insulin sensitivity in type 2 diabetes mellitus patients, but little is known on L-arginine effects on these variables in nondiabetic patients with stable cardiovascular disease (coronary artery disease). We evaluated the effects of long-term oral L-arginine treatment on endothelial dysfunction, inflammation, adipokine levels, glucose tolerance, and insulin sensitivity in these patients. Sixty-four patients with cardiovascular disease previously submitted to an aortocoronary bypass and not known for type 2 diabetes mellitus had an oral glucose load to define their glucose tolerance. Thirty-two patients with nondiabetic response were eligible to receive, in a double-blind randomized parallel order, L-arginine (6.4 g/d) or placebo for 6 months. An evaluation of insulin sensitivity index during the oral glucose load, markers of systemic nitric oxide bioavailability and inflammation, and blood flow was performed before and at the end of the treatment in both groups. Compared with placebo, L-arginine decreased asymmetric dimethylarginine levels (P < .01), indices of endothelial dysfunction, and increased cyclic guanosine monophosphate (P < .01), L-arginine to asymmetric dimethylarginine ratio (P < .0001), and reactive hyperemia (P < .05). Finally, L-arginine increased insulin sensitivity index (P < .05) and adiponectin (P < .01) and decreased interleukin-6 and monocyte chemoattractant protein-1 levels. In conclusion, insulin resistance, endothelial dysfunction, and inflammation are important cardiovascular risk factors in coronary artery disease patients; and L-arginine seems to have anti-inflammatory and metabolic advantages in these patients.
Summary
| Aminoacid | Carnitine | Creatine | Choline | Coenzyme Q |
| Arginine | X | X | . | . |
| Methionine | X | X | X | X |