STRUCTURE
Pathway
FUNCTION


SUPPLY
SYNTHESIS
creatine biosynthesis

Creatine biosynthetic pathway. HA, h′epatic artery; PV, portal vein; HV, hepatic vein; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; GAMT, guanidinoacetate N-methyltransferase; AGAT, L-arginine:glycine amidinotransferase; GAA, guanidinoacetate acid.
Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo, 2009
DIET
CATABOLISM
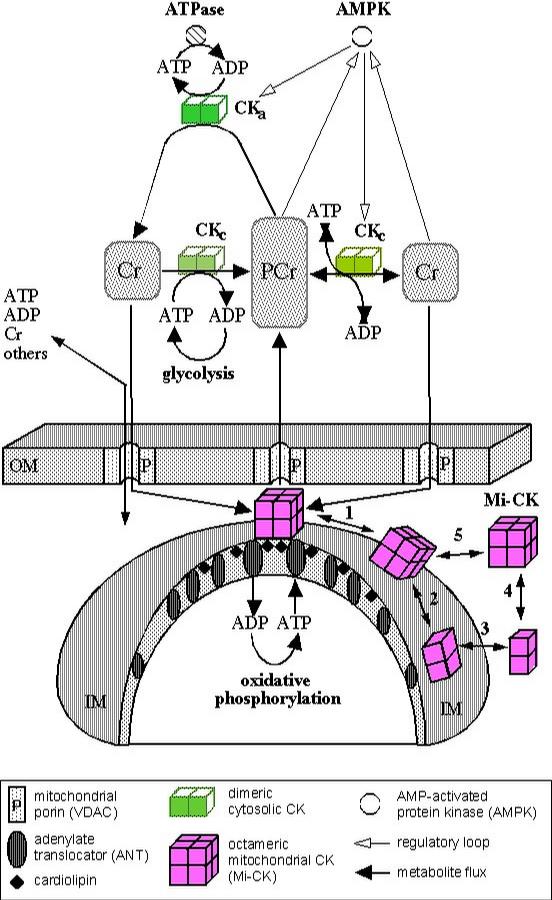
Multiple Functions of Creatine Kinase for Cellular Energetics: a Scientific Rationale for Creatine Supplementation
Impaired muscle uptake of creatine in spinal and bulbar muscular atrophy, 2016
Objective
The aim of this study was to explore the pathomechanism underlying the reduction of serum creatinine (Cr) concentrations in spinal and bulbar muscular atrophy (SBMA).
Methods
We evaluated blood chemistries, motor function, and muscle mass measured by dual‐energy X‐ray absorptiometry in male subjects with SBMA (n = 65), amyotrophic lateral sclerosis (ALS; n = 27), and healthy controls (n = 25). We also examined the intramuscular concentrations of creatine, a precursor of Cr, as well as the protein and mRNA expression levels of the creatine transporter (SLC6A8) in autopsy specimens derived from subjects who had SBMA and ALS and disease controls. Furthermore, we measured the mRNA expression levels of SLC6A8 in cultured muscle cells (C2C12) transfected with the polyglutamine‐expanded androgen receptor (AR‐97Q).
Results
Serum Cr concentrations were significantly lower in subjects with SBMA than in those with ALS (P < 0.001), despite similar muscle mass values. Intramuscular creatine concentrations were also lower in with the autopsied specimen of SBMA subjects than in those with ALS subjects (P = 0.018). Moreover, the protein and mRNA expression levels of muscle SLC6A8 were suppressed in subjects with SBMA. The mRNA levels of SLC6A8 were also suppressed in C2C12 cells bearing AR‐97Q.