DEFINITION
The terms CLEFT LIP and CLEFT PALATE indicate generally the presence of a labial or palatine cleft. The cleft and lip palate is a face’s malformation that can afflict the upper lip, the alveolar process, the hard palate and the soft palate.
It is possible classify the different types of cleft lip and cleft palate in:
Cleft lip with cleft palate (CLP) (35-55%)

Cleft lip without cleft palate (CL) (20-30%)

Cleft palate isolated (CPO) (35-45%)

These groups of malformations, even if are associated anatomically, are two different entities under several outlooks: embryological, epidemiological, genetic and etiopathogenetic.
By definition CLP is a unilateral or bilateral cleft of the upper lip, that can extend to the nostril’s floor, with or without palate’s cleft. Instead the clef palate is a cleft of the hard and/or the soft palate, with extension and breadth variable.
EPIDEMIOLOGY
The CLP is a quite frequently pathology: it affects a child for every 500-700 born (1,57/1000). We can notice high values in Northern Europe; whereas in the rest of Mediterranean Europe there are lower prevalence rates. We find :
- the highest frequency of malformation in native Americans: 4/1000 born. The prevalence is high also among oriental, especially among Japanese and Chinese;
- relatively low prevalence in African population: 3/100000 born.
The more accredited hypothesis about the different incidence in the ethnic groups is that the malformation is related to the complex polygenic that adjusts the face’s size. The anatomical variability of the face’s shape in the different ethnic groups might influence the different predisposition to the cleft. Between the genders the clef afflicts more the men.
Even if in the most of the people the malformations characterized by cleft are isolated, in the 30% of children with CLP there are others congenital faults (syndromic form).
CLP's epidemiology, Dr Roberto Corelli 2007.
EMBRYOLOGY
To understand the CPL’s appearances is indispensable to know the embryogenetic processes of the constitution of the face’s different parts.
FACE'S DEVELOPEMENT
The face develops from five sketches, the facial processes, that appear on the primitive mouth’s walls or “stomodeum” during the forth week of embryonic development.
- the frontal process (in the top) represents the stomodeum’s roof.
- the mandibular symmetrical processes (down) represent the stomodeum’s floor.
- the maxillary symmetrical processes (lateral) represent the stomodeo’s side.
From the forth week of development the facial processes are subjected to several morphogenetic movements that change their shapes and sizes to melt in preconcerted areas, originating the definitive structure of the mouth.
The lip and the cheeks are invaded by the mesenchyme derived from the second brachial arch. From this arch originate also the lip and cheeks’ muscle that are innervated by the facial nerve.
face's development
PALATE'S DEVELOPEMENT
The palate develops from two structures: the primary palate or palatine lamina and the secondary palate. The secondary palate derives from the posterior parts of maxillary processes and is formed by two laminas that during the sixth week of development, grown obliquely downward originating the palatine processes.
During the period between the seventh and the ninth week, the tongue’s sketch migrates downward and the palatine processes change direction and migrate one toward the other on a horizontal plane. At the tenth week the palatine processes merge together and with the nasal septum and the primary palate to form the secondary palate. In the middle of the secondary palate it is possible to see the fusion’s line of palatine processes (palatine raphe) and in the median line the incisor foramen, point of contact between the primary and the secondary palate. The primary palate and the secondary palate’s anterior part, as a result of membraneous ossification’s process, originate the hard palate whereas the secondary palate’s posterior part doesn’t ossify and form the soft palate and the uvula.

CONGENITAL MALFORMATIONS
The face develops from the confluence of the five brachial arches so a lack in the fusion of any pair of these processes produce a cleft in one o more parts of the face.
We can classify the different types of cleft in several ways.
- anterior: in front of the incisor foramen
- posterior: behind the incisor foramen
The anterior cleft can be suddivided in:
- unilateral clef lip: only one face’s side is implicated

- bilateral cleft lip:both face's sides are implicated

Under the malformation’s gravity:
- simple lip cleft: the malformation afflict only the lip without crossing the gum

- completed lip cleft: the malformation extends from the nostrils to the incisor foramen

More images:

A:incompleted unilateral cleft lip
B:unilateral cleft of the lip; alveolus and palate
C:bilateral cleft of the lip, alveolus and palate
D:isolated cleft palate
Cleft lip
Cleft palate
Unilateral cleft lip and palate
The CL is caused to a non-fusion of the facial processes due to:
- insufficient migration of the neural crest’s cells
- insufficient proliferation of the neural crest’s cells
The CP is caused to a non-fusion of the palatine processes due to:
- insufficient and unfit cellular proliferation
- the palatine processes can’t change direction at the right time
- the palatine processes even if are in touch can’t fuse
- the head’s excessive widening
CAUSES
Understood the ways by whicth the structures of nose, lip, jawbone and palate develop, it’s necessary to considerate the factors that, interfering with embriogenetic process, are responsible the wrong fenotipe’s manifestation. In the 30% of cases the malformative event is linked to a genic predisposition while, in the remaining 70% of cases, the esogen factors are the most importante cause that bring to the appearence of clefted fenotipe.
GENETIC CAUSES
Four categories of genes are results suggestive of a genetic susceptibility to OCs. They are:
- Genes expressed in a particular area of the embryo or in a particular period of the palatine arch development, such as the transforming growth factors alpha and beta, like TGF alpha, TGF beta 2, TGF beta
- Genes having biological activities linked to the OC's pathogenesis without direct involvement, e.g. the retinoic acid receptor (RARA), the methylenetetrahydrofolate reductase receptor (MTHFR) and the folic acid receptor (FOLR1)
- Genes or locus identified in experimental animals as the homeotic genes MSX-1 and MSX-2
For more information: Orofacial Cleft 1
Environment and genetics in the etiology of cleft lip and cleft palate with reference to the role of folic acid 2000
EXTERNAL FACTORS
The external factors exercitate on the fetus a teratogenic effect, in fact are able to cross the placenta and produce neonatal defects. To have a teratogenic effect they must act between the fifth and the eighth week of pregnancy. After that period, the facial’s anatomic structures are made and they’re no more at risk.
External factors are:
- Drugs:
- Anticonvulsives: neuropharmacological agents may be teratogenic to human beings. Because many neuropharmacological agents function through neurotransmitter mechanisms, neurotransmitters could affect embryonic development. There is evidence that neurotransmitters function as biological signals in palate development. Like the CNS, palate shelf reorientation is modulated by a diverse range of neurotransmitters. For example, shelf reorientation is stimulated by acetylcholine and serotonin and inhibited by GABA.
Diazepam's effects on non sindromic cleft lip with or without cleft palate, 2011
- Non-steroidal anti-inflammatory drug:many non-steroidal anti-inflammatory drugs (NSAIDs) (including sulphasalazine, sulindac, indomethacin, naproxen, salicylic acid, ibuprofen, piroxicam and mefenamic acid) were found to be competitive inhibitors (with respect to folate) of enzymes that act on folate methabolism.
Inhibition of folate-dependent enzymes by non-steroidal anti-inflammatory drugs
Aspirin-alcol interaction in he production of cleft palate, 1995
- Smoke:both serum folate and red blood cell folate are lower in pregnant women who smoked than in pregnant women who did not smoke. There is an important gene environment interaction between methylenetetrahydrofolate reductase gene activity and tobacco exposure on serum folate levels. Lower levels of serum folate may account for the higher rate of miscarriage, stillbirth, abruptio placentae, and fetal anomalies that are observed in pregnant women who smoke. Pregnant women who smoke may benefit from higher doses of folic acid periconceptionally.
Drinking and smoking association: a bad habit?
Others risck factors could be the the teratogenic effects of some infective deseases, even if the mechanism through which they act are still unknown:
- Rubella
- Chickenpox
- Parotitis
- Toxoplasmosis
- Influenzal diseases.
The non sindromic cleftpalate is the most frequent craniofacial deformation in the human population, but its aetiology is very complex and it’s object of study yet. The fact that it has a familiar recurrence, improves the hypothesis that genetic factors play a keyrole in this congenic malformation’s insurgence.
FOLIC ACID AND CLP
A periconceptional assumption of folic acid is demonstrated to exercitate a protection effect on the defect of neural tube’s closing, like spina bifida. Preliminal evidency suggest the idea that folic acid may reduce the non sindromic orofacial schisis’s risk too. Further evidences indicate that high ematic levels of homocysteine are associated to orofacial schisis’s development.
Dose-dependent effect of folic acid on the prevention of orofacial clefts,1999
Oral clefts and vitamin supplementation,2001
It’s important to underline the existence of some risk factors that can improve the folic acid’s requirement or reduce the uptake, like the assumption of some drugs, alcool beyond a specific genes’ variations involved in the folate methabolism, like MTHFR gene, that codificate for metilentetraidrofolate reductase enzyme, the folate receptors (FOLR) and TCN2 gene, that codificate for 2-transcobalamine.
Resuptive scheme of folate's methabolic way:
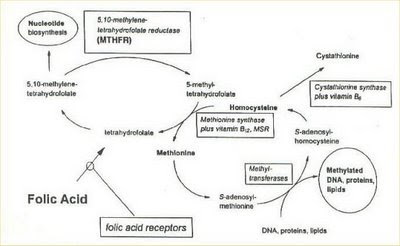
- MTHFR: the 1p36 region of this gene codificate for the methylentetraidrofolate reductase. This enzyme plays a keyrole in the folate methabolic way, converting the 5,10-methyletratraidrofolate to 5-methyltetraidrofolate.
Polimorphysm:
- C677T: this variation is translate in a proteic product having the aminoacidic sostitution Ala222Val. The gene with T variation codificates for a thermolabile form of the enzyme with reduce activity, responsible for low emetic levels of folate and high emetic level of homocysteine.
C677T variant form at the MTHFR gene: a risk for mothers?, 2001
- A1298C: it seems instead to codificate for a enzyme that act on the folate methabolism only during the periods of most request of the one (like pregnancy and embryogenesis).
- FOLATE RECEPTORS: FOL1 and FOL2. These receptors are indispensabile for the intercellular tran sport and for the folate internalization’s process in mathernal cells, that makes them available for the fetus. Recent studies have excluded these receptors’ involvement in the aetiology of LPS (scapoli, 2005a).
- TCN2 (2-transcobolamine): the transcobolamine are plasma’s acid glycoprotein that have an importal role in the B12 vitamin’s (cobolamine) transport, from the intestinal lumen to other cellular districts, through the haematic circle. Exist three different types of transcobolamines, TCN1, TCN2 and TCN3, that differ from each other for the oligosaccharidic component and for the morphologic and functional specificity. The concrete cobolamine carriers in all the organism are TCN2 molecules.
- MTR and MTRR: they’re responsible for the 50% of rimethylation to methionin of the formed homocystein. The MTRR catalyzes the reduction and methylation of cobolamine to methylcobolamine, restoring the methionine sintase’s activity. Polymorphism to genes codifying for enzyme involved in the folate/homocystein’s metabolic way, like MTHFR, MTR and MTRR are another risk factor of predisposition to schysis.
CONCLUSIONS
the daily folate and B group vitamines’ assumption, during the periconceptional period, higly reduce onset’s risk of neural tube’s defect and concenic malformations. . A study by Van Rooji and colleagues,2003 has demonstrated that it exist an association between LPS and plasmatic concentration of folate, homocysteine and B6 and B12 vitamines, underlying the probable correlation between the plasmatic levels of these molecules and the mutations’ onset on genes involved in the folate’s metabolic way.
In a study by Rosenquist, 2007 it has been underlined that 65 genes of known function that were altered by homocysteine. The largest set of effected genes (19) included those with a role in cell migration and adhesion (the most important are MAP1B, ROCK2, SEMA6D). Other major groups were genes involved in metabolism (13); DNA/RNA interaction (11); cell proliferation/apoptosis (10); and transporter/receptor (6).Regardless of the way that the homocysteine signal is transduced, the key to understanding how homocysteine may disrupt cellular function, and ultimately how it perturbs embryonic development, is to identify the genes that are modulated downstream of elevated homocysteine. The most well-described developmental effect of elevated homocysteine is its ability to disrupt derivatives of the neural ectoderm.
Folic acid and orofacial cleft: a review of the evidence,2009
Simona Astesano
Genta Elisa