The molecular pathways as a basis for rational therapy of renal tumors.
Introduction
The RCC is in the western world, about 3% of adult malignancies.
Since the 70s it has seen a steady increase in its incidence, in part associated with improved imaging techniques that allow for the diagnosis of these tumors in the earliest stage. The incidence increases with age, being very low before 40 years, reaching a peak around 60 years with a ratio man: woman ratio of 2:1. Survival is correlated with tumor stage at diagnosis: it is poor for metastatic tumors (12% at 5 years), while it is high (about 80%) in patients with renal tumors as single location. We identified several risk factors for the development of renal cell carcinoma: of these, as well as some kidney’s diseases, dietary factors, environmental and genetic factors are particularly relevant.
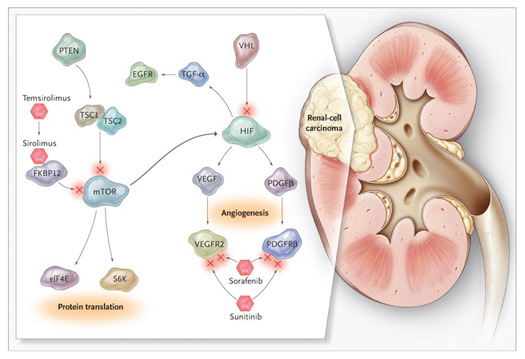
Molecular basis of pathogenesis of renal cell carcinoma
Renal cell carcinoma originates from the transformation of tubular epithelial cells of the kidney; there are both sporadic and hereditary forms, including those inscribed in the von Hippel-Lindau syndrome , Birt-Hogg- Dubé (BHD) in the hereditary papillary renal cell carcinoma (HPRC).
The RCC is therefore not classifiable as a single disease, but rather is represented by a set of location types in renal cancer with different histological features, clinical outcomes and molecular pathogenesis.
The conventional or clear cell renal cell carcinoma is the form with the higher rate (75-80%); it occurs mainly in sporadic forms, but there are familiars too, defined by the VHL syndrome. In both cases, the molecular pathogenesis is due to inactivating mutations of the VHL tumor suppressor gene, which encodes a component of the ubiquitin-ligase responsible for ubiquitination of Hypoxia inducible Factors 1α e 2 α. This process is sensitive to tissue oxygen concentrations. In fact, under conditions of normoxia, the HIF-α is hydroxylated at proline residues by specific enzymes, prolyl-hydroxylase activated by oxygen free radicals; it leads to recognition by VHL, which makes the HIF-α start the proteasomal degradation. In presence of hypoxia (which inhibits the prolyl-hydroxylase) or inactivating mutations of VHL, HIF-1α 2α accumulate, respectively dimerize with HIF-1β and 2β, and bind the Hypoxia Responsive Elements (HRE) present in the genes’s promoters regulating cell survival, angiogenesis and metabolism. The role of VHL in the pathogenesis of renal cell carcinoma, however, seems to be more complex and related in part to events beyond the control of HIF. For example, it was shown that renal cell carcinoma with inactivating VHL mutations responds to treatment with the Hepatocyte Growth Factor (HGF) showing increased motility and invasive capacity; in fact, the removal of the negative feedback of VHL against the signalling pathway of MET, a receptor tyrosine kinase (RTK) whose ligand is the HGF, seems to favor the redistribution of α-catenin to the cell nucleus, where it acts as a transcriptional coactivator.
Papillary renal cell carcinoma or chromophilia (incidence 10-15%) also occurs in sporadic or familiar forms. There are two subtypes, and in the pathogenesis of type 1 are involved inactivating mutations of the MET oncogene. Recent studies have shown that the fumarate, the substrate of FH, is a competitive inhibitor of prolyl hydroxylase that starts HIF-1α and HIF-2α to the recognition by VHL and proteasomal degradation. Accumulation of fumarate in presence of mutations in the FH gene therefore results in an intracellular accumulation of HIF and increased expression of genes regulated by it.
Chromophobe renal cell carcinoma (incidence 4-6%) is a variant with a better prognosis than the first two forms; the molecular mechanisms involved in its development are little known. It may arise in the context of BHD syndrome, caused by mutation of tumor suppressor gene FLCN: the follicle, the protein encoded by this gene seems implicated in the signaling pathway protein mammalian target of rapamycin .
Role of HIF in the pathogenesis of renal cell carcinoma
As we said before, it appears that a common mechanism underlying the pathogenesis of renal cell carcinoma is the alteration of cellular responses to hypoxia. Hypoxia, understood as the limitation of oxygen carried at cells and tissues, is a condition that typically occours in human tumors. In fact, during the process of growth of the tumor cells located within the same they move away gradually from the blood vessels, suffering a reduction in the supply of oxygen and nutrients to the induction of angiogenesis and the formation of new intratumoral vessels, but because of abnormal characteristics of tumor vasculature, persist even at this stage of hypoxic areas within the tumor. Hypoxia profoundly influences the biological behavior, prognosis and response to therapy of human cancers: in fact, it may constitutes a selective pressure that can confer a growth advantage to cells resistant to low concentrations of oxygen. The resistance to hypoxia is usually from gene mutations that cause activation of survival signaling pathways, specific metabolic adaptations, and induction of angiogenesis aimed at improving the supply of oxygen, these characteristics are themselves associated with a more aggressive and metastatic power, as well as resistance to chemotherapy and radiotherapy.
A key role in the response of mammalian cells to hypoxia is played by transcription factors of the HIF family. In kidney tumors, and VHL mutations found in clear cell carcinoma than those of FH detected in papillary type 2 leads to increased levels of HIF, gene mutations FLCN characteristics of chromophobe cell carcinomas are related to abnormal activation of the mTOR, which also leads to induction of HIF. To confirm this, overexpression of HIF has been documented in all histological subtypes of renal cell carcinoma, including those with papillary and chromophobe cells, in particular, immunohistochemical analysis of early renal lesions have demonstrated increased levels of both HIF-1 that HIF-2, and recent evidence suggests that in renal clear cell carcinomas of defective VHL, HIF-2 may have an even more important role of HIF-1. In fact, despite these two factors have the same sequence of binding to promoters, it appears that HIF-1 is more involved in the induction of genes regulating metabolism, while those in HIF-2 in growth and angiogenesis.
Since the induction of HIF is a feature common to different types of renal cell carcinoma, the study of target genes of these transcription factors explain most of the molecular features observed in these tumors. These include growth factors such as Transforming Growth Factor (TGF) - α and their receptors such as MET; proangiogenici factors such as Vascular Endothelial Growth Factor (VEGF) and Platelet Derived Growth Factor (PDGF)-B; chemokines and their receptors as Stromal Cell Derived Factor (SDF) -1, CXC Chemokine Ligand-motif 12 (CXCL12), Chemokine Receptor CXCR4, transporters and enzymes of glucose metabolism such as GLUT-1, lactate dehydrogenase, pyruvate dehydrogenase kinase, pH-regulators such as carbonic anhydrase IX and XII. Each of them plays a key role in some aspects of the development and progression of renal tumors location: For example, because of renal cell carcinoma cells express the Epidermal Growth Factor Receptor (EGFR), an RTK that can interact with TGF - α induction of the autocrine growth factor generates circuits that stimulate the proliferation and survival of cancer cells. In addition, the induction of VEGF, PDGF-B and other factors proangiogenici explains the marked vascularity and the high dependence dall'angiogenesi of renal cell carcinoma. Increased expression of MET, the aforementioned chemokines, and the matrix metalloproteinase promotes cell motility and invasion processes. The induction of the enzymes colicky, together with the inhibition of enzymes of the Krebs cycle and the process of oxidative phosphorylation, leads to significant changes in the metabolic processes that govern the use of nutrients by the cells. Likewise, the more that inhibition of HIF in renal cell carcinoma is effective interference with the proteins induced by this class of transcription factors that regulate key processes of proliferation, angiogenesis and bioenergetics.
Role of HIF in the induction of angiogenesis
The tumor angiogenesis, or formation of new blood vessels that ensures an adequate supply of oxygen and nutrients to tumor growth, a process essential for tumor growth and the development of distant metastases. Several molecules are involved in 'tumor angiogenesis, including PDGF-B, angiopoietin (Ang), bFGF, interleukin-8 (IL-8) and other cytokines. However, VEGF is the main mediator of angiogenesis in human cancer: its overexpression is involved in the "angiogenic switch" is associated with increased intratumoral tumor vasculature and correlates with poor prognosis, increased risk of nationalization goal and resistance to treatment in different types of cancer.
In mammals, the VEGF family includes five members: VEGF-A (described simply as VEGF), VEFG-B, VEGF-C, VEGF-D, VEGF-E and Placental Growth Factor (PIGF). VEGF is able to interact with three different RTK, called VEGF Receptors: VEGFR-1 (Flt-1), VEGFR-2 (KDR) and VEGFR-3 (Flt-4). The VEGFR-2, whose expression is mainly restricted to vascular endothelial cells, is the main signal transducer for angiogenesis, both physiological and pathological. The VEGFR-1 has a high affinity for VEGF-A, but its specific ligands are VEGF-B and PIGF, but its tyrosine kinase activity is relatively weak, and in fact it does not stimulate significantly the proliferation of endothelial cells. The VEGFR-3, whose ligands are VEGF-C and VEGF-D, regulates lymphangiogenesis: its expression in adults seems to be limited to the lymphatic endothelial cells. Closely connected to the system VEGF / VEGFR is that of PDGF and its receptors. The PDGF family comprises four members, called PDGF-A,-B,-C and-D, capable of joining together to form homo-and hetero-dimers. The signal transduction depends on the interaction with two different TKR, the PDGFR-α and-β, expressed by endothelial cells and pericytes, perivascular supporting cells pupils required for the stabilization and maturation of blood vessels.
In the context of tumor angiogenesis, induction of VEGF and PDGF by HIF promotes proliferation and migration of endothelial cells, and also contributes alk recruitment of pericytes and stromal cells can amplify the response proangiogenica. In addition, the VEGF and PDGF contributes to the development and progression of human cancers not only indirectly through the induction of tumor angiogenesis, but also directly, by regulating the proliferation and survival of cancer cells: it is known that both VEGFR that PDGFR is also expressed by tumor cells of renal cell carcinoma by activating autocrine circuits that support their proliferation.
The inhibition system VEGF / VEGFR and PDGF / PDGFR is now a strategy in the treatment of renal cell carcinoma: the main tools you need this treatment approach are monoclonal antibodies (mAb) capable of sequestering the ligands and prevent interaction with receptors and inhibitors of tyrosine kinase (TK1) can antagonize the phosphorylation of the receptors. Indeed, biological agents used clinically to treat kidney cancer include bevacizumab, a humanized variant of a monoclonal antibody neutralizing VEGF, and TK1 multiple sunitinib, sorafenib, pazopanib and dresses, active on VEGFR, PDGFR, c-kit and , with the exception of pazopanib, even on Flt-3.

Role of HIF-1 in the regulation of metabolism
HIF-1 is able to reprogram the metabolism of glucose in response to hypoxia. This is done primarily through the induction of transporters that mediate the uptake of glucose into the extracellular enzymes of the colicky operating the conversion of glucose to pyruvate and lactate dehydrogenase A (LDH-A), which converts pyruvate to lactate. At the same time, the induction of pyruvate dehydrogenase kinase 1 (PDK-1), which phosphorylates and inactivates pyruvate dehydrogenase, prevents the conversion of pyruvate to acetyl CoA and its entry into the Krebs cycle. In addition, HIF1 induces a change in the composition of the complex of cytochrome c oxidase and inhibition of mitochondrial biogenesis, thereby reducing the consumption of oxygen in the process of mitochondrial respiration. Taken together, these events to determine the level of tumor cells a metabolic shift known as the Warburg effect, characterized by a predominance of anaerobic glycolysis to the process of oxidative phosphorylation. Several studies have shown that this phenomenon is associated with poor prognosis in advanced renal cell carcinoma.
The signaling pathways involved in regulating the levels of HIF
The levels of HIF-1α and-2α in the cell are finely regulated by the balance between the process of synthesis and degradation that in turn influenced by the microenvironment in which the cell is located. We have already talked about how the system VHL regulates the degradation of HIF-α level in a proteasome-dependent oxygen concentrations, and how alterations of this system leads to an increase in the levels of constitutive HIF. A crucial role in regulating the synthesis of HIF-1α is played instead of the protein mTOR, a central regulator of protein synthesis and involves different types of extracellular signals.
The mTOR signaling pathway

mTOR is a protein that abnormally takes its name from its target, the antibiotic rapamycin. It is a serine / threonine kinase that can integrate multiple intracellular signals for the regulation of cell growth, metabolism, and angiogenesis and can interact with other proteins to form two types of complexes, called mTORC1 and mTORC2. mTORC1 can be activated by growth factors such as VEGF, PDGF and EGF via the PI3K (phosphoinositide-3-kinase) / Akt, also warns nutritional signals, such as deprivation of glucose and amino acids, due to the protein AMPK (5 'AMP- activated protein kinase). mTORC1 is responsible for most of the functions performed by mTOR, including control of protein synthesis by activation of ribosomal p70S6 kinase (S6K1) and the elimination of 4E-binding protein (4E-BP), protein-binding factor translation initiation. Through this mechanism, mTORC1 is responsible for the induction of HIF-1α.
Although no activating mutations were found neither mTOR nor gene amplification in human tumors have been demonstrated alterations of one or more elements of its signaling pathway in a wide spectrum of cancers. One way of adjustment of components of mTOR is in fact an alteration in the processes of growth and cell metabolism. The mTOR signaling pathway is often hyperactive in renal cell carcinoma, for example as a result of inactivating mutations of the PTEN tumor suppressor gene, which encodes a phosphatase that acts as a negative regulator of the PI3K/Akt path. The presence of alterations of the mTOR signaling pathway is an important prognostic factor in patients with renal cell carcinoma.
Based on these findings, and preclinical and clinical studies that have shown antitumor activity, two inhibitors of mTOR rapamycin analogs, temsirolimus and everolimus, are now used in the treatment of renal cell carcinoma. The effectiveness of these agents in this model, cancer seems to depend mainly on the ability to interfere with downstream activation of tumor angiogenesis. An important aspect seems to be the ability of mTOR inhibitors to interfere with all components of the tumor vasculature, including smooth muscle cells and pericytes, in addition, inhibition of mTOR mediated knows everolimus seems to be able to avoid the induction of VEGF characteristically reported after inhibition of VEGF signaling pathway. Finally, mTOR may be a way out other than blocking signal transducers such as VEGFR / PDGFR, therefore its inhibition is able to enhance the effectiveness of other anticancer therapies used in renal cell carcinoma. This is the case of TK1 sunitinib and sorafenib, which are being studied for combinations / sequences with mTOR inhibitors could potentially maximize the therapeutic effects and prevent the development of resistance phenomena.
Conclusions
The RCC is defined by a set of types of cancer in renal localization characterized by several histological, molecular pathogenesis and clinical outcomes. The molecular mechanisms at the origin of the different forms of renal cell carcinoma, however, due largely to a change in the cellular response to hypoxic conditions; in this process, HIF family of transcription factors play a crucial role in inducing the expression of a number of genes involved in the activation of signaling pathways for survival, and inducing metabolic adaptations in the process of tumor angiogenesis. Inhibition of HIF, realized, for example, by interfering with the way of mTOR, which regulates the synthesis in mammalian cells, inhibition of target gene products of these transcription factors are now effective therapeutic strategies in treatment of renal cell carcinoma.
References
- Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer journal 2008; 14:288-301.
- Smaldone MC, Maranchie JK. Clinical implications of hypoxia inducible factor in renal cell carcinoma. Urol Oncol 2009; 27:238-45.
- Dhote R, Thiounn N, Debrè B, Vidal Trecan G. Risk factors for adult renal cell carcinoma. The Urologic Clinics of North America 2004; 31:237-47.
- Linehan WM, Pinto PA, Bratslavsky G, et al. Hereditary kidney cancer: unique opportunity for disease-based therapy. Cancer 2009;115:2252-61.
- Lonser R, Glenn G, Walther MM. von Hippel-Lindau disease. Lancet 2003; 361:2059-67.