POSITIVE AND NEGATIVE EFFECTS OF THALIDOMIDE'S USE
MOLECULE

(RS)-2-(2,6-diosso-3-piperidinil)-1H-isoindol-1,3(2H)-dione
NEGATIVE EFFECT: THALIDOMIDE TERATOGENECITY
The journey of thalidomide was started in 1956 when it was marketed as a non-barbiturate sedative agent. It was considered as a wonder drug that provided safe and sound sleep and hence, used to cure morning sickness in pregnant women. Thalidomide: Chemistry, therapeutic potential and oxidative stress induced teratogenicity.2012
However, thalidomide exposure during the first trimester of pregnancy caused multiple birth defects. Defecsts such as malformation of the limbs, ears, eyes,internal organs and central nervous system were reported. Thalidomide most frequently induced two categories of limb defects: phocomelia and amelia. Phocomelia is a disorder in which the limbs consist of a truncated or absent zeugopod and a nearly intact autopod. Amelia is marked by the complete absence of or more limbs. Ear malformations induced by thalidomide varied from microtia (the underdevelopment of the outer ear) to anotia ( the absence of the outer ear). Ocular anormalies such as microphthalmia ( an abnormally small eye or eyes) were also observed. Defects of the internal organs including kidney and heart malformation were frequent. Moreover, autism and mental retardation were reported in some of affected individuals, even though the incidence was low. Malformation of the limbs was the most commonly reported thalidomide-induced birth defects.
Studies of thalidomide teratogenicity have been conducted for 50 years. There are more than 30 hypotheses to explain how thalidomide causes limb malformation. For example, it has been suggested that thalidomide may intercalate with GC box of specific gene promoter for insulin-like growing factor 1. In this regard, Igf 1 is crucial for limb development and growth. However, there is little or no in vivo experimental evidence to support the GC box hypothesis. On the other hand, two models of thalidomide teratogenicity are widely supported: the anti- angiogenesis and oxidative stress models. In fact thalidomide inhibited fibroblast growth factor 2 induced angiogenesis/ vasculogenesis in rabbits, so it was supposed that the distruption of vasculogenesis contributed to limb malformation because blood circulation is crucial for limb development. Metabolic breackdown is required for thalidomide to exert its anti-angiogenetic activity. Thalidomide is bio-transformed by liver cytochrome P450 into its hydroxylated products. Ample experimental evidence also supports the oxydative stress model of thalidomide teratogenicity. Exposure to thalidomide resulted in a elevation or reactive oxygen species. Thalidomide induced programmed cell death by the upregulation of BMPs following the genaration of ROS. BMPs block signaling mediated by Fgfs as well as by Akt and Wnt.
However the direct target of thalidomide is CRBN, that is a protein made of 442 amino acids. It forms an E3 ubiquitin ligase complex with DDB1, Cul4A, and Roc1, which is important for the expression of fibroblast growth factor 8, an essential regulator of limb development. Expression of a drug binding-deficient mutant of cereblon suppressed thalidomide-induced effects in zebrafish and chicks. This suggests that thalidomide downregulates fibroblast growth factor 8 expression and induces limb malformation by binding to wild-type cereblon, inhibiting the function of the associated E3 ubiquitin ligase. Identification of a primary target of thalidomide teratogenicity. 2010

POSITIVE EFFECT
THALIDOMIDE AND MULTIPLE MYELOMA
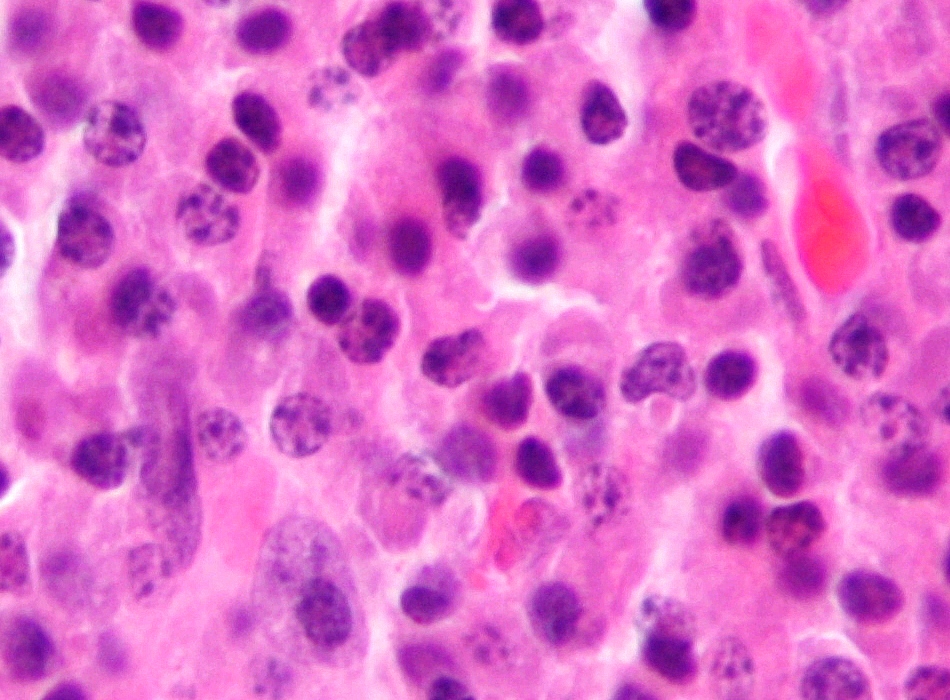
Multiple myeloma is characterized by an overproduction of malignant plasma cells in the bone marrow.
Thalidomide and its analogue lenalidomide are recently approved for the treatment of multiple myeloma. These agents modulate expression of a wide range of cytokines such as interleukin 2 and interferon gamma that stimulate T cells and natural killer cells to destroy MM cells, and they downregulate expression of cytokines such as IL-6 and tumor necrosis facotor alfa that contribute to angiogenesis. Lenalidomide, compared with thalidomide, demonstrates improved activity and a better safety profile. Lenalidomide is effective in patients who relapse or are refractory to thalidomide, and, compared with thalidomide, is associated with less peripheral neuropathy but a similar risk of thromboembolic events.
The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. 2012
THALIDOMIDE AND PEDIATRIC REFRACTORY CROHN'S DISEASE
Crohn’s disease is a chronic transmural inflammatory bowel disease that may involve any part of the alimentary tract from mouth to anus, especially the distal ileum and colon. It may also accompany various intra- and extra-intestinal complications during its clinical course. In addition to causing serious clinical symptoms and complications, its chronic nature can adversely affect the quality of life of patients. A recent study proves that thalidomide could be usefull in the treatment of Cronh’s deasease. It was a retrospective study of six patients with refractory Crohn’s disease, who visited the Children’s Hospital of Fudan University in 2006-2010. Patients administered thalidomide in the evening at a starting dose of 2.0 mg/kg per day, which was increased to 2.5-3.0 mg/kg per day or decreased to 1.5 mg/kg per day, according to the individual patient’s response to the drug. Patients were assessed at baseline, at weeks 2, 8 and 12, and then every three months for the following parameters, including physical examination, laboratory analyses and pediatric CD activity index (PCDAI) scoring. Endoscopies were repeated at six months after administration of thalidomide. Its side effects were intensely monitored during follow-up. CD was defined as refractory when standard induction therapy with high-dose intravenous steroids failed to induce remission either at diagnosis or during subsequent relapse. Efficacy was defined as thalidomide induced and maintained remission and mean time to achieve clinical remission. These positive effect of thalidomide are related to the inhibition of the synthesis of tumur necrosis factor alfa that plays a key role in physiopathology of Crohn’s desease. Treatment of pediatric refractory Crohn's disease with thalidomide. 2011
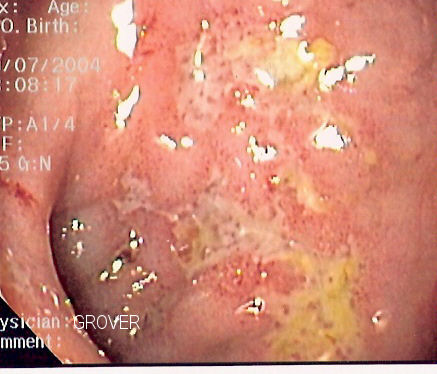
THALIDOMIDE AND REFRACTORY BLEEDING DUE TO GASTROINTESTINAL ANGIODYSPLASIAS
Gastrointestinal bleeding and chronic ferropenic anemia resulting from vascular lesions of the digestive tract sometimes pose a difficult therapeutic challenge due to the location and multiplycity of such lesions. This causes the deterioration in the quality of life of patients. The presence of high levels of VEGF promotes aberrant angiogenesis and formation of angiodysplasias with a vascular endothelium lacking in smooth muscle cells and therefore prone to ruptures. Thalidomide is a drug with a potent anti anti-angiogenic effect which inhibits VEGF and reduces its level significantly. This property of thalidomide was demonstrated by a prospective study of a series of multi-trasfused patients with gastrointestinal bleeding and severe ferropenic anemia who did not respond to endoscopic argon plasma coagulation treatment or somatostatin analogue therapy. All patients were informed about thalidomide treatment and gave their informed consent to take part in the study. For each the following parameters were considered: age, sex, underlying pathology, previous therapies, dose and duration of thalidomide treatment, evolution of haemoglobin concentration during treatment and transfusions required as well as adverse side effects of the treatment. The diagnosis of gastrointestinal angiodysplasias was obtained through upper and lower gastrointestinal endoscopy. The study included 12 patients, 7 males and 5 famales. The dose of thalidomide administered was 200 mg/24 h and the estimated initial duration of treatment was 4 mounths for all patients. However, the treatment was discontinued after 3 days in one patient due to gastrointestinal intolerance to the drug and after one week in another patient due to fever and thrombophlebitis after cat scratchin. Another patient showed signs compatible with anoxal sensitive polyneuropathy which prompted withdrawal of thalidomide. The symptoms resolved once the treatment had finished. None of the patients required transfusions during the study period. After two months after onset of therapy the haemoglobin concentration showed a significant increase which continues until the end of therapy.
In addition endoscopic capsule examination shows a reduction in the number, size and colour of vascular lesions in patients receiving thalidomide treatment, and control of haemorhage during a mean follow up period of 2-3 years without necessity of transfusions. In general, the adverse side effects such as fatigue, peripheral neuropathy, skin rush, are not severe. Morover, as thalidomide acts in the origin oh these lesions through VEGF inhibition, it prevents the development of future angiodysplasias. So thalidomide is a recent and promising therapeutic option in patients with a gastrointestinal bleeding and anaemia secondary to vascular malformations. Thalidomide in refractory bleeding due to gastrointestinal angiodysplasias. 2012
Torino, 15/07/2012