INTRODUCTION
As the interface between humans and their environment, the skin plays a critical role in protecting internal organs against pathogen entry and in reducing the loss of body fluid.
The efficiency of this mechanism is achieved by the cornified layer and the strong cohesion between adjacent keratinocytes.
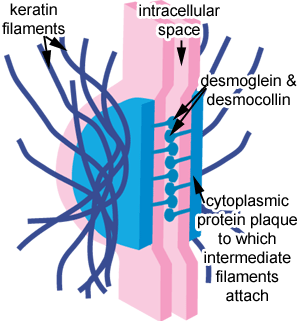
Desmosomes are molecular complexes of cell adhesion proteins and linking proteins that attach the cell surface adhesion proteins to intracellular keratin cytoskeletal filaments.
The cell adhesion proteins of the desmosome, desmoglein and desmocollin, are members of the cadherin family of cell adhesion molecules. They are transmembrane proteins that bridge the space between adjacent epithelial cells by way of homophilic binding of their extracellular domains to other desmosomal cadherins on the adjacent cell. Both have five extracellular domains, and have calcium-binding motifs.
The extracellular domain of the desmosome is called the Extracellular Core Domain (ECD) or the Desmoglea, and is bisected by an electron-dense midline where the desmoglein and desmocollin proteins bind to each other. These proteins can bind in a W, S, or λ manner.
On the cytoplasmic side of the plasma membrane, there are two dense structures called the Outer Dense Plaque (ODP) and the Inner Dense Plaque (IDP). These are spanned by the Desmoplakin protein.The Outer Dense Plaque is where the cytoplasmic domains of the cadherins attach to desmoplakin via plakoglobin and plakophillin. The Inner Dense Plaque is where desmoplakin attaches to the intermediate filaments of the cell.
The SSSS (generalized form of the bullous impetigo) constists in a loss of keratinocytes cell-cell adhesion due to the action of Exfoliatives Toxins (Ets). Today three isoforms of exfoliatives toxins have been found (ETA,ETB,ETD) in Staphylococcus Aureus.
They are glutamate specific serine proteases that specifically cleave a single peptide bond in the extracellular region of human desmoglein 1 (Dsg1, a desmosomal cadherin type cell-cell adhesion molecule).
ETs are species-specific and they facilitate bacterial invasion into mammalian skin.
CLINICAL ASPECTS
Bullous impetigo is a highly contagious bacterial disease that primaily affects children, mainly newborn, and it consists in a rapid progression of flaccid bullae which rupture and become covered with crusts. Staphylococcus Aureus cultures producing ETs can be isolated from the contents of intact bullae.

In SSSS (known as Ritter's disease) there are flaccid blisters and superficial denudation or desquamation. Most affected individuals are children under 5 years of age,immunocompromised adults and IRC patients. Mucous membranes are not affected in SSSS. Cultures obtained from intact bullae of SSSS are usually negative for staphylococcus aureus producing ETs. It seems that toxins produced at a distant focus of infection (e.g. laryngopharynx) reach the skin through the bloodstream as a result of toxemia, causing SSSS eruptions.
ETs STRUCTURE
Studies appears to show that ETA (predominant form in Europe and USA) is chromosomally located, whereas ETB gene is carried on plasmids. Furthermore eta gene is encoded in the genomic sequence of a temperate phage, and phage infection converts ETA non producing strains in into ETA producers. ETA and ETB comprise 242 and 246 respctevely aminoacids and share 40% of aminoacids homology.
Comparisons of the deduced aminoacids sequences of ETA and ETB showed that they share primary aminoacids homology with staphylococcal V8 protease (a trypsin-like protease). Crystal structure analyses revealed that their three-dimensional structures resemble those of glutamate-specific serine proteases, including the presence of catalytic triad (His, Asp, Ser).

CLINICAL MANIFESTATION OF SSSS COMPARED TO PEMPHIGUS FOLIACEUS
The understanding of ETs mechanism came in 2000 when similarities with Pemphigous Foliaceus were noted. In PF IgG autoantibodies against Dsg1 disrupt the intercellular adhesion of keratinocytes and cause epidermal blistering. In humans four isoforms of Dsg have been identified. Their extracellular region contains five cadherin repeats separated by calcium-binding domains.
Similar pathophysiological features are shared in SSSS and PF. Both cause predominantly cutaneous lesions despite the involvement of mucous membranes, including oral mucosa.
ETs and PF serum IgG from patients both cause intraepidermal blistering and identical histopathological changes when injected in neonatal mice.
Immunofluorescence studies of the skin of mice injeted with ETA revealed that the surface of keratinocytes stained poorly for Dsg1, whereas Dsg3 staining remained at the normal level. Immunoblot analyses of epidermal extracts of these mice and of Dsg1 and Dsg3 expressing cultured keratinocytes incu was shown to cleave the extracellular domain of human and mouse Dsg1 in a dose dependent fashion, but not Dsg3.
Unlike ETA and ETB, ETD-producing strains of Staphylococcus Aureus were isolated mainly from other manifestations of infection than bullous impetigo or SSSS, such as cutaneous abscesses, foruncles and finger pulp infections. This observation suggests that ETs plays a more general pathogenic role not only in bullous impetigo or SSSS, but also in a broad spectrum of cutaneous infections.
Recent studies show that all three ET isoforms cleaved human and mouse Dsg1 at one position after glutamic acid residue 381, between extracellular domain (EC) 3 and EC4. This cleavage site is located in the putative calcium binding site of Dsg1, and the removal of calcium ions block the cleavage of Dsg1 by both ETA and ETB. Substituting the predicted catalytic serine by an alanine results in complete loss of cleaving activity.
Other data suggests that the catalytic triad of ETA is inactive in the absence of any substrate, and inacccessible to serine protease inhibitors.
It's established that ETs as distinct serine proteases recognize and hydrolyze efficiently and specifically their substrates. Hydrolysis depends on the appropriate aminoacids sequence and on substrate conformation.

In the late 1990s, several attempts were made to determine wheter purified ETs indeed have superantigenic activity. Recombinant ETA and ETB produced in a superantigen-free strain of Staphylococcus Aureus stimulate the proliferation of human peripheral blood mononuclear cells (PBMCs) in a dose-dependent fashion. However the mitogenic potencies of these toxins were 100 times less than those of toxic shock syndrome toxin-1 (TSST-1) and staphylococcal enterotoxins (Ses).
These findings suggest that the superantigenic activities of staphylococcal ETs are not involved in the pathogenic process of SSSS and bullous impetigo and they don't support the superantigen theory in the pathogenesis of this disease.
Recent but non-conclusive studies confermed that ETs cleave Dsg1 in a species-specific manner. It appears that staphylococci acquired the ability to produce ETs, which facilitate pecutaneous bacterial invasion of certain mammals by species-specific cleavage of specific keratinocyte cell-adhesion molecules.
The identification of the substrate of staphylococcal ETs has provided insight as to why the toxins cause epidermal separation predominantly in the superficial epidermidis.
It remains to be determined how exfoliative-toxin-producing staphylococci circumvent the cornified layer of the epidermis or the follicular infundibulum and enters the superficial epidermis.
FUTURE PERSPECTIVES
Currently, therapy for bullous impetigo and SSSS in humans consists mainly in antibiotic therapy.
Whereas staphylococci carrying et genes have long been susceptible to B-lactam antibiotics, the frequency of eta-, etb- and etd-positive strains of MRSA in clinical or carriage isolated is increasing, raising the possibility that antibiotic-resistant, esfoliative-toxin-producing staphylococci can become pathogens in bullous impetigo or SSSS.
The development of novel alternate therapies may rapidly become an urgent necessity. The generation of neutralizing antibodies that efficiently inhibit the reaction between enzyme and substrate will provide a novel therapeutic option for SSSS cuased by MRSA.
REFERENCES
Staphylococcal exfoliative toxins: ‘‘Molecular scissors’’ of bacteria that attack the cutaneous defense barrier in mammals, Koji Nishifuji, Motoyuki Sugai, Masayuki Amagai
http://www.nextbio.com/b/search/article.nb?id=17582744
Dermatologia e Venereologia
Amerio P.L., Bernengo M.G., Calvieri S., Chimenti S., Pippione M., Aricò M., Aste N., Borroni G., Leigheb G., Micali G., Nunzi E., Offidani A.M., Tulli A.
2009, 2° edizione, casa editrice Minerva Medica
Harrison's Principles of Internal Medicine, 18° edition
Dan L. Longo, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, J. Larry Jameson, Joseph Loscalzo.