The bladder estrophy is usually included in bladder exstrophy-epispadias-complex (BEEC): an
anterior midline defect with variable expression that affects the infraumbilical abdominal wall including the pelvis, urinary tract, and external genitalia. It ranges from isolated epispadias (E), to classic bladder exstrophy (CBE), to its most severe form, cloacal exstrophy (CE) also known as OEIS complex (omphalocele, exstrophy of the bladder, imperforate anus, spinal defects).
Prevalence at birth ranges from 1/30,000 for CBE to 1/200,000 for CE. There is a greater proportion of affected males than females, ranging from 1.8:1 to 6:1. While the etiology of BEEC remains unknown, strong evidence exists linking its development to genetic effects. The etiological association of genetic determinants is supported by the discrepant male to female ratio; a 400-fold increase of the recurrence risk for offsprings of affected individuals;
observations of rare multiplex families; and much higher concordance rates (62% vs. 11%) among monozygotic as compared to dizygotic twins. Its non-Mendelian pattern of segregation and the incomplete concordance among MZ twins implies a complex trait model of inheritance.

EMBRYOGENESIS OF THE URINARY TRACT
The urinary tract is formed by three components:
1) the kidneys which, originate from the metanephros
2) the upper urinary tract (ureters, cups, tubes), which originate from the mesonephros
3) the lower urinary tract (urethra and bladder) that originate from the urogenital sinus (endoderm).
The development of the urogenital sinus starts from the fourth week when the cloaca is divided into a anterior portion, the urogenital sinus precisely, and a posterior portion from which is going to born the rectum. The cloaca during the four week goes to occupy a ventral position forming the abdominal anterior wall.
The urogenital sinus at the top extends towards the allantois, while at the bottom is temporarily closed from the urogenital diaphragm, which will break during the seventh week, putting it in communication with the amniotic fluid.
The development of the bladder takes place between the fourth and seventh week, while there is the septation of cloaca, the walls of the urogenital sinus expand and incorporating the bottom of the ducts of Wolf and than the ureters; so the ureters come out directly into the urogenital sinus in two new orifices. Subsequent migration of the ureters and Wolf ducts product that the mesonephros trapped into Wolf ducts takes a triangular shape and it will be create trigone of bladder.
At the end of the third month the splanchnic mesoderm, lining the urogenital sinus, goes to build the muscles of the bladder and the allantois is going to close becoming the urachus.
ETIOPHATOGENETIC THEORIES ABOUT THE ORIGIN OF BLADDER EXTROPH
The embryogenesis of exstrophy-epispadias complex is a subject that has been a source of conjecture and controversy. It is also known that the human embryo does not pass through a stage of development that corresponds to exstrophy bladder. Hence, it is impossible from embryologic point of view to explain the bladder exstrophy as a result of simple developmental arrest. Numerous theories have been proposed to explain the etiopathogenesis of exstrophy bladder:
1 Hp states that the anterior rupture of the embryonic bladder is caused by the abnormal retention of fluid. However, this does not explain the commonly associated anomalies of genital and musculoskeletal systems.
2 Hp proposed that the abnormally large cloacal membrane would act as a wedge to the developing structure of the lower abdominal wall.But even if cloacal membrane is a normal embryologic structure in that area, and even if it is abnormally large, it should not act as a wedge. A cloacal membrane normally is an unstable structure lacking mesoderm, and it has a strong tendency to disintegrate. Hence, it can not have wedge effect.
3 Hp have suggested the anatomical basis of a common embryological origin for epispadias, bladder and cloacal exstrophy. It suggest that the first anomaly could be the lack of rotation in the pelvic ring primordia. It is a fact that the pelvic ring primordia doesn’t rotate; however, it is the result of the higher origin of the genital tubercle. The event of the rotation of pelvic ring primordia occurs later in a chronological order (seventh week) than during the origin of genital tubercle (fourth week).
4 Hp is a new theory that propose that abnormal origin of the primordia of genital tubercle in a cephalad position than the normal will result in the wedge effect, and hence, it will interfere with the medial migration of the mesoderm as well as the midline approximation of mesodermal structures in the lower abdominal wall and prevent the lengthening of the body stalk, which is observed in newborn babies whose umbilical cord is inserted at the apex of the exstrophied bladder. This hypothesy is supported by the observation that in all cases of exstrophy, there is a normally pigmented skin bridge interposed in between the penile shaft (clitoris in female) and the scrotum (labia majora in female) dissociating these two structures.
HISTOPATHOLOGY
Normal histology :Bladder layers are mucosa (urothelium, lamina propria, discontinuous muscularis mucosa), muscularis propria, adventitia, serosa/peritoneum at dome.
Urothelium:
● Formerly called transitional epithelium since intermediate between nonkeratinizing squamous and pseudostratified columnar epithelium
● 5-7 cell layers thick in contracted bladder, 2-3 cells thick in distended bladder; lines renal pelvis, ureters, bladder, most of urethra but not distal urethra
● Superficial urothelium (umbrella cell layer) is single layer of umbrella cells, which are large and elliptical with abundant eosinophilic cytoplasm and often binucleation or prominent nucleoli.
Lamina propria:
● Located between the mucosal basement membrane and the muscularis propria, is thinner at the trigone and bladder neck
● Contains loose to dense connective tissue, variably sized blood vessels that may be close to epithelium, lymphatics, variable adipose tissue
● Discontinuous muscular mucosa, usually associated with intermediate-sized arteries and veins; only 5% of bladders have well developed, continuous muscularis mucosa
● It is advisable to separate lamina propria invasion into above or beneath muscularis mucosae since the former is more likely to behave as a Ta tumor
Muscularis propria:
● Consists of inner longitudinal, circular and outer longitudinal layers of thick muscle bundles, may also contain adipose tissue between muscle fascicles, paraganglia
● Muscle layers are distinct only near bladder neck and in the remaining areas the longitudinal and circular layers mix freely without definite orientation.
● Muscularis propria may be greatly thickened if obstruction to urine flow develops
Von Brunn’s nests (proliferative cystitis):
● Reactive proliferative change present in 85-95% of bladders; frequency increases with age; more common at the trigone
● Nests of cytologically benign urothelium in lamina propria; nests have regular spacing, extend to same horizontal level at base of proliferation
● May or may not have continuity with surface epithelium; inflammation is usually minimal; stromal reaction is absent

Histopathologic analisys in bladder extrophy
The pathologic findings of hematoxylin-eosin (HE) and Azan staining of the bladder showed highly increased collagen fibers in the submucosal layer and a decrease in the smooth muscle layer. The increase in collagen fibers in other organs causes loss of distensibility in the arteries or increased stiffness of the heart. Considering these facts, the pathologic findings in our patient suggest the compliance of the bladder was reduced. The decrease in the smooth muscle layer also indicated a decrease in detrusor contractility and compliance. Immunohistochemistry of the bladder showed a decrease in neuron cells, and the kit-positive interstitial cells in the submucosal layer indicated reduced conveyance of intrabladder stimulation to the spinal cord, leading to insensitivity of urinary storage. In addition, fewer neuron and kit-positive cells were found in the submucosal layer, indicating that the bladder sensation was insufficient and closer attention to frequent urethral catheterization would be required to preserve the upper urinary tract. Thus, the pathologic findings of the bladder contribute to speculation on future bladder function and to the management of the neuropathic bladder.
ENVIROMENTAL FACTORS
Environmental factors have an important role during the period of gastrulation , a process by which the bilaminar embryonic disc is converted to trilaminar embryonic disc.It is the beginning of morphogenesis and is significant event occurring during the third week. Gastrulation begins with the formation of primitive streak at about fifteenth day of embryonic life. The primitive streak is a midline proliferative region of the epiblast where the cells may break free from the epithelium and migrate beneath the epiblast to form the intra embryonic mesoderm. Gastrulation errors can be explained as errors in proliferation, migration and subsequent differentiation of the intra-embryonic mesoderm resulting in defective morphogenesis.
Environmental factors can again be classified into intrinsic and extrinsic. Intrinsic factors include the local environment around the developing embryonic disc (i.e.uterine endometrium and the cavities of the embryo). In first week of life, the blastomeres derive their nourishment, in part, from stores laid down in the cytoplasm of the primary oocyte and from tubal and uterine secretions. In second and third week of life, the embryonic disc is dependent on nutrients obtained from the fluid filled cavities of the amnion, the celom and the yolk sac. These fluids contain products arising
because of absorption by the trophoblast from the lysed uterine tissues and extravasated maternal blood. However, these sources of supply are much diminished and inadequate at an early stage in development. It therefore becomes imperative that some other source should be available at an early stage. This involves formation of placenta and establishment of fetal circulation. By the end of the third week, the primitive cardiovascular system is established and the heart begins to beat so that the blood now circulates. Thus, the third week of fetal life is a challenging period, when a transition of source of nutritional supply to the developing embryo is taking place. During the
third week, the existing nutritional supply starts diminishing and the fetal circulation is yet to start. Incidentally, it is the same period when gastrulation process is going on. The diminishing supply of nutrients is bound to affect the deeper tissues (mesoderm) more than the layers towards the fluid filled cavities (the ectoderm and the endoderm) and hence the vulnerability of the mesoderm. Support to this hypothesis comes from the fact that increase in the incidence of multiple congenital
malformations is recognized in the children of diabetic mothers. It is known that diabetes causes tissue starvation and hence the uterine endometrium is unable to provide optimal nutritional support.
Although often discussed as a cause the extent to which the extrinsic factors e.g. alcohol, smoking, medication, toxins can affect the embryo at this stage is also a matter of investigation, because extrinsic environmental factors come into play only after placentation and the establishment of fetal circulation resulting in intimate contact of the embryo with the maternal blood.
TEMPORAL-SPATIAL PROTEIN EXPRESSION IN BLADDER TISSUE
Identifying developmental proteins could lead to markers of bladder progenitor cells, which could be used to investigate bladder diseases. Understanding normal bladder development is of the utmost importance for developing strategies to treat congenital and acquired conditions that affect the bladder. Differentiation of the epithelium and mesenchyma into mature urothelium and smooth muscle appear to occur in an orderly sequence and in a specific pattern.
Embryologically the bladder forms from the urogenital sinus, which is an extension of the hindgut, which is an endodermal derivative. Based on the fact that the 2 structures arise from the urogenital sinus, which is an endodermal derivative, colud be study the temporal-spatial protein expression that induced the differentiation of signalling necessary for the presence of bladder structure. These different proteins are turned on and off during endodermal maturation until a certain stem cell exists that forms into urothelium and, if surrounded by the proper microenvironment, ultimately into an organized bladder.
Bladder urothelium has the capability to differentiate into different cell types, as evidenced by squamous metaplasia or cystitis glandularis. This suggests that stem cells exist in the urothelium and signals from the local tissue environment dictate their ultimate fate in organogenesis.
Between the factors that characterize the bladder progenitors cells there is Foxa2 , which is highly expressed in these cells, and after the bladder epithelium matures it is no longer expressed. It is supported by an indirect relationship between urothelial maturation (uroplakin positivity) and decreased Foxa2 expression.
For the smooth muscle differentiation has an important role Shh , a paracrine signal from urothelium to the surrounding mesenchyma. High levels of Patched , the receptor for Shh, are present in the mesenchyma surrounding the urothelium and decreased Patched expression is seen at the bladder periphery. Therefore, smooth muscle is inhibited at high concentrations of Shh and induced at low concentrations. Furthermore, Shh mutants have severe anorectal malformations, including hypoplasia of the bladder lumen and wall.
IMPORTANT ROLE OF p63
p63 is a p53 homologue required for cutaneous development that is expressed in immature squamous epithelium and reserve cells of the cervix.
The strong rationale for studying p63 as a candidate gene for human BEEC is based on its coordinating function during anogenital modeling and epithelial cell differentiation in the developing female mouse urogenital tract.
In the absence of p63, the ventral urothelium is neither committed nor differentiated, while the dorsal urothelium is both committed and differentiated. It has been proposed that p63 is required for the maintenance of ‘stemness’ of all stratified epithelia, or required for the very fundamental steps of commitment of and differentiation processes in stratified epithelia. The p63 (KET/p40/p51/p73L) gene encodes at least eight protein isoforms realized by alternative splicing and alternative initiation of transcription , alternate promoter usage results either in the presence (TA) or absence (ΔN) of a
classical transactivation domain.
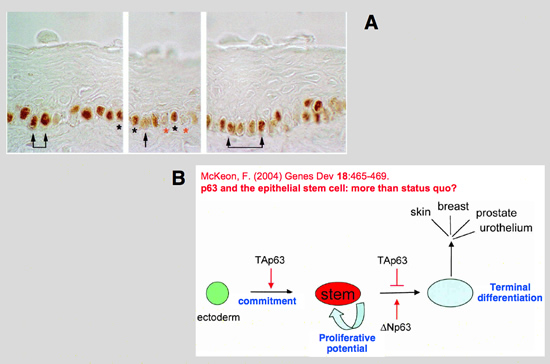
By using antibodies, discriminating between only TA and ΔN forms, it has been established that ΔNp63 is the predominant isoform expressed throughout the bladder with a preferential expression in the ventral bladder urothelium during early development. It has also been shown that ΔNp63 is required for ventral specification in zebrafish. Loss of ΔNp63 results in reduction of ventral (non-neural) ectoderm, while ΔNp63 overexpression expands the ventral ectoderm.
To obtain a comprehensive overview of the transcriptional activity of p63 during the development of the urogenital system and external genitalia, Ching et al ( p63 (TP73L) a key player in embryonic urogential development with significant dysregulation in human bladder exstrophy tissue; 2010 ) performed whole mount in situ hybridization (WISH) on mouse embryos at gestational days 9.5-12.5 with particular emphasis on the region of the cloaca and the genital tubercle
These analysis of the p63 expression pattern in midgestation phase mouse embryos clearly showed the spatiotemporal correlation of p63 transcriptional activity with the critical phase of urogenital development.
Loss of p63 in mice was shown to cause limb and craniofacial defects, multiple malformations in urogenital development, and defects in the formation of the abdominal wall, resulting in exstrophy of the bladder. As causes for these pleiotropic defects, failures in epithelial to mesenchymal signalling as well as an antiapoptotic role of p63 were described. p63 expression in the cloacal and uretral epithelium suggests that p63 is necessary for proper function as a source of patterning or proliferation signals on the mesenchyme of the adjacent lateral plate and
genital tubercle mesoderm. The loss of p63 activity in this tissue can thus lead to the observed evelopmental defects that are a prerequisite for bladder exstrophies. ΔNp63 is required for epithelial development and formation of stratified epithelia, the lack of all or some of these isoforms might be involved in the formation of urogenital system malformations. ΔNp63 protein induces expression of the extracellular matrix component Fras1, required for maintaining the integrity of the epidermaldermal interface at the basement membrane. Mutations in human FRAS1 have been causally linked to classical Fraser syndrome, an autosomal-recessive defect, also known as Cryptophthalmos-Syndactyly syndrome. CFS shows phenotypic overlap with BEEC in that umbilical hernia (omphalocele), microphallus in males along with cryptorchidism, vaginal atresia or bicornuate uterus in females as well as diastasis of symphysis pubis in both genders are frequently observed.

MAIN GENES INVOLVED IN BLADDER EXSTROPHY
Many studies have been conducted to identify the genes involved in BEEC ( Genome-wide expression profiling of urinary bladder implicates desmosomal and cytoskeletal dysregulation in the bladder exstrophy-epispadias complex; 2011 ). The studies discovere that the two most down-regulated genes in BEEC, desmin and desmuslin are involved in desmosome mediated cell-cell adhesion and cytoskeletal architecture. Intriguingly, the sixth most overexpressed gene was desmoplakin, the most abundant desmosomal protein. Further findings indicate that p63, PERP, SYNPO2 and the Wnt pathway may also contribute to BEEC etiology. The desmosome, a rivet-like cellular junction structure, is primarily located in epithelial and muscle cells and is specialized for cell-cell adhesion. In addition to maintaining the structural integrity of tissues by resisting shear forces, desmosomes are critical for the formation of organs and tissues during embryogenesis. The molecular blueprint of the functional desmosome varies between tissues, but
generally consists of 3 principal components including desmosomal cadherins (desmocollins and desmogleins), armadillo type of junctional proteins (plakophilins and placoglobin), and plakin proteins (desmoplakin, plectin, envoplakin and periplakin). Plakin proteins serve as an anchor of the IF. As an integral part of the cytoskeleton IF confer resistance to physical stress by distributing mechanical forces throughout a tissue and participate in the formation of supracellular scaffolding that maintains the integrity of tissues. In addition to these classical mechanical functions of the desmosome, there is evidence that mechanical forces transmitted along the length of the IF to the nucleus regulate gene expression.
Keratin proteins constitute a major component of the IF in all epithelia and 3 type I cytokeratin genes, KRT10, KRT18 and KRT19, were found to be up-regulated in the EB. Even more intriguing was that the 2 most down-regulated genes in EB, DES and DMN encode muscle-specific IF proteins that directly interact with the C-terminal domain of desmoplakin, encoded by DSP, the sixth most overexpressed gene in EB samples. Evaluation of DES in the MAMEP database reveals that it is expressed in the differentiating somites of the trunk, the cloacal membrane and the ureteric cord, in accordance to its function in muscle development. These findings indicate that abnormal formation and/or anchoring of the IF to the desmoplakin component of the desmosome may be the specific cellular defect in BEEC.
Another gene observed is PERP, a tetraspan protein that is a direct p63 downstream target. The studies show that it is overexpressed in human bladder extrophy. Intriguingly, PERP, specifically localizes to the desmosomes where it extends through the plasma membrane to link neighboring cells.


Another highly up-regulated gene was WNT5A. WNT5A is strongly expressed in the paraxial and lateral mesoderm in the caudal end of the embryo, and is essential in the caudal extension of the body axis as well as the formation of the external genitalia. The expression of WNT5A is induced by Gli2. Interestingly, p63 expression in the cloacal endoderm is also up-regulated by Gli2, suggesting that both WNT5A and p63 are regulated by a common upstream pathway. Further support for a causal interplay of WNT5A in the expression of BEEC, arises from the physical overlap of the RRM2 gene with a region of suggestive linkare because it is up-regulated in EB tissues, and it functions downstream of ß-catenin as an inhibitor of Wnt signalling.
