.
.
.

Daboia (Daboia, Wikipedia) is a monotypic genus of venomous Old World viper. The single species, D. russelii (commonly known as Russell's viper), is found in Asia throughout the Indian subcontinent, much of Southeast Asia, southern China and Taiwan. The species was named in honor of Patrick Russell (1726–1805), a Scottish herpetologist who first described many of India's snakes; the name of the genus is from the Hindi word meaning "the lurker." Apart from being a member of the big four snakes in India, Daboia is also one of the species responsible for causing the most snakebite incidents and deaths among all venomous snakes on account of many factors, such as their wide distribution and frequent occurrence in highly-populated areas.
Daboia's venom is, therefore, extremely dangerous and toxic, affecting initially the zone surrounding the bite, and then the whole organism; it causes local swelling, local necrosis, coagulopathy, neurotoxicity, nephrotoxicity, cardiac effects and myotoxicity. However, because of his interactions with factors of coagulation FV and FX (so his ability to induce thrombosis), this venom can also be used in laboratory tests to find clotting problems or the presence of particular anticoagulant antibodies.
Envenomation
The snake threatened raises the first third of the body and produces a hiss louder than that of any other snake. When striking from this position he can exert a great force, and the bite may be a snap, or they may hang on for many seconds.
Envenomation symptoms begin with pain at the site of the bite, immediately followed by swelling of the affected extremity (in 92% of the bittens). Bleeding is a common symptom, especially from the gums and in the urine, and sputum may show signs of blood within 20 minutes post-bite. There is a drop in blood pressure, and the heart rate falls (3-12%). Blistering occurs at the site of the bite, developing along the affected limb in severe cases. Necrosis is usually superficial and limited to the muscles near the bite (8.9%), but may be severe in extreme cases. Vomiting and facial swelling occur in about one-third of all cases. Renal failure also occurs in approximately 25% of untreated bites. Severe disseminated intravascular coagulation also can occur in severe envenomations (77% of victims develop prolonged WBCT). Severe pain may last for 2–4 weeks. Death from septicaemia, kidney, respiratory or cardiac failure may occur 1 to 14 days post-bite or even later.
Acute Myocardial Infarction following a possible direct intravenous bite of Russell’s viper (Daboia russelli), PubMed Central, 2012
Venom
The quantity of venom produced by individual specimens is considerable. Reported venom yields for adult specimens range from 130–250 mg to 150–250 mg to 21–268 mg.
Two well-known procoagulant enzymes found in the Russell's viper venom have been characterized: factor X activator (RVV-X), a type IV metalloprotease, and factor V activator (RVV-V), a single chain serine protease.
RVV-V (RVV-V, PENTAPHARM) is a 29 kDa single chain serine protease, consisting of 236 amino acid residues and 6% carbohydrate. RVV-V specifically cleaves the single peptide bond between Arg1545 and Ser1546, resulting in activation of factor V, a key component of the hemostatic system which acts as a co-factor in prothrombinase complex. Factor Va accelerates factor X-catalyzed prothrombin conversion by 300,000-fold.
(Phylogenetic analysis of serine proteases from Russell's viper (Daboia russelli siamensis) and Agkistrodon piscivorus leucostoma Venom, PubMed Central, 2011)
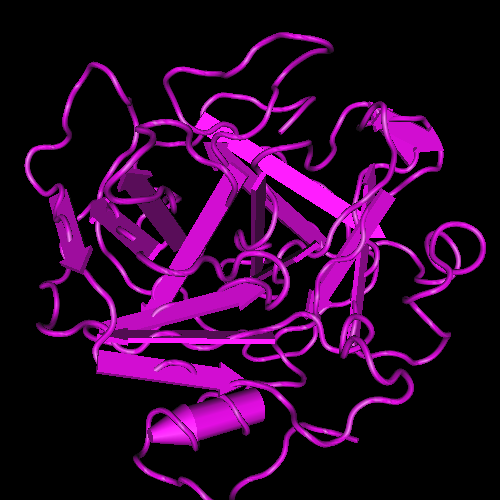
RVV-X (RVV-X, PENTAPHARM) is a dimer of two peptide chains with a molecular weight of 60’000 g/mol each. Activation of factor X by RVV-X strictly depends on the presence of calcium ions; it is a potent activator of factor X, is a well characterized proteinase which specifically activates factor X as a result of a single cleavage at the same internal Arg-Ile bond in factor X as do factors IXa and VIIa.
(Coagulation Factor X Activating Enzyme from Russell’s Viper Venom (RVV-X), The Journal of Biological Chemistry, 1992)

Snake Venom Proteases, Haematologic Technologies Inc.
Factor V (FV) is one of the key components of the blood coagulation cascade. Human FV is a single-chain glycoprotein of 2190 amino acid residues and consists of six domains, A1, A2, B, A3, C1, and C2. FV circulates in the blood as a precursor molecule and is converted into the active form, FVa, after consecutive cleavage of the three peptide bonds, Arg709–Ser710, Arg1018–Thr1019, and Arg1545–Ser1546, by thrombin or activated factor X (FXa). These cleavages remove the highly glycosylated B domain from FV, resulting in the exposure of the binding site for FXa. FVa acts as an essential cofactor in thrombin generation: the rate of the prothrombin-to-thrombin conversion by FXa is enhanced by several orders of magnitude in the presence of FVa and Ca2+ on phospholipid membranes.
Venom serine proteinases (SVSP) belong to the MEROPS peptidase family S1, subfamily S1A (chymotrypsin-A subfamily). SVSPs interfere mostly with the hemostatic system upon envenomation. Despite significantly high sequence identity (50–70%), SVSPs display high specificity toward distinct macromolecular substrates. RVV-V is an FV-activating SVSP isolated from Russell’s viper venom (Russell's Viper Venom Serine Proteinase, Rvv-v in Complex With the Fragment (Residues 1533-1546) of Human Factor V, NCBI, 2011). RVV-V, which consists of 236 amino acids, cleaves only the Arg1545–Ser1546 bond of FV and does not cleave the other two thrombin-susceptible bonds. Therefore, cleavage of FV by RVV-V does not release its B domain. However, FV cleaved by RVV-V acquires the ability to bind to FXa and shows the same level of procoagulant activity as FV activated by thrombin. While thrombin acts on numerous proteins associated with hemostasis other than FV, no protein substrate other than FV has been identified for RVV-V to date. Prolonged incubation of RVV-V with factor VIII, fibrinogen, prothrombin and FX showed no apparent effects on either the structures or activities of these proteins.
Structural basis of coagulation factor V recognition for cleavage by RVV-V, ScienceDirect, 2011
Venom metalloproteinases (SVMPs) differ in their domain structure. Some enzymes comprise only the metalloproteinase domain, others have disintegrin-like and high cysteine domains and others present, besides these domains, an additional lectin-like subunit. All of them are zinc-dependent enzymes with highly similar zinc binding environments. Some metalloproteinases induce hemorrhage by directly affecting mostly capillary blood vessels; as a consequence, these cells undergo a series of morphological and functional alterations in vivo, probably associated with biophysical hemodynamic factors such as tangential fluid shear stress. Eventually, gaps are formed in endothelial cells through which extravasation occurs. In addition to hemorrhage, venom metalloproteinases induce skeletal muscle damage, myonecrosis, which seems to be secondary to the ischemia that ensues in muscle tissue as a consequence of bleeding and reduced perfusion. Moreover, venom metalloproteinases participate in the degradation of extracellular matrix components and play a relevant role in the prominent local inflammatory response that characterizes snakebite envenomations, since they induce edema, activate endogenous matrix metalloproteinases (MMPs) and are capable of releasing TNF-alpha from its membrane-bound precursor.
dRVVT (Dilute Russell's viper venom time)
The limited specificity of RVV-V toward FV, and of RVV-X toward FX, among the components of blood clotting has made it an extremely useful tool in the investigation of FV of FX both in the laboratory and for diagnostic purposes. It is used in the in vitro diagnostic test dRVVT (Dilute Russell's viper venom time), based on the ability of the venom of the Russell's viper to induce thrombosis. The coagulant in the venom directly activates factor X, which turns prothrombin into thrombin in the presence of factor Va and phospholipid. In the dRVVT assay, low concentrations of both Russell's viper venom and phospholipid are used to give a standard clotting time of 23 to 27 seconds.

Lupus Anticoagulant
The diluite Russell's viper venom is also useful to identify the presence in the blood of lupus anticoagulants (LA); these are immunoglobulins that bind to phospholipids and proteins associated with the cell membrane, and are prothrombotic agents (the presence of Lupus anticoagulant antibodies precipitates the formation of thrombi in vivo).
The disease name is a misnomer, it derives from their properties, in vitro, of interferring with phospholipids utilized to induce in vitro coagulation. In this particular case, it can be noticed a prolonged clotting time of 30 seconds or greater during dRVVT, and this gives evidence of the diagnostic importance of the Russell's viper venom in detecting patogen antibodies.

LA antibodies are involved in Antiphospholipid syndrome (APS), an autoimmune disease in which antiphospholipid antibodies react against proteins that bind to anionic phospholipids on plasma membranes.
In APS there are antibodies binding to protein S, which is a co-factor of protein C (involved in proteolytically inactivating proteins FVa and FVIIIa). Thus, anti-protein S antibodies decrease protein C efficiency. Antibodies also bind to Annexin A5, which forms a shield around negatively-charged phospholipid molecules, thus reducing their availability for coagulation. So anti-annexin A5 antibodies increase phospholipid-dependent coagulation steps.
LA antibodies bind to prothrombin, increasing its cleavage to thrombin, its active form, and to Apolipoprotein H (also known as β2glycoprotein I), an apolipoprotein normally assuming an anti-coagulation activity in serum. When LA antibodies binds to Apo-H, they depress its activity, and the final result is a facilitation of thrombogenesis.
The Lupus anticoagulant antibodies are those that show the closest association with thrombosis, and those that target β2glycoprotein I have a greater association with thrombosis than those that target prothrombin. The subset of those that bind Apo-H and alter its activity are considered different from antibodies that bind thrombin and are called anti-apolipoprotein antibodies. In autoimmune disease, anti-apolipoprotein antibodies (Anti β2 glycoprotein I antibodies) strongly associate with thrombotic forms of SLE (Systemic lupus erythematosus) and sclerosis.

Antiphospholipid syndrome can increase the risk for DVT (Deep venous thrombosis), while the most common arterial event is stroke; furthermore, it can cause pregnancy-related complications (miscarriage, pre-eclampsia, placental infarctions, stillbirth), thrombocytopenia, heart valve disease, and livedo reticularis. There are also associations between antiphospholipid antibodies and headaches, migraines, and oscillopsia.
Acute prognostic factors for post-thrombotic syndrome in children with limb DVT: a bi-institutional cohort study, PubMed, 2013