Warfarin vs NOACs…
Limitations and Consequences of Warfarin
- Slow onset of action: Overlap with a parenteral anticoagulant
- Genetic variation in metabolism: Variable dose requirements
- Multiple food and drug interactions: Frequent coagulation monitoring
- Narrow therapeutic window: Frequent coagulation monitoring.
Moreover, even when VKAs (vitamin K antagonists) are administered to such patients, the level of anticoagulation is often outside the therapeutic range. These problems highlight the need for new oral anticoagulants that overcome the limitations of VKAs and are effective, safe and convenient for long-term administration.
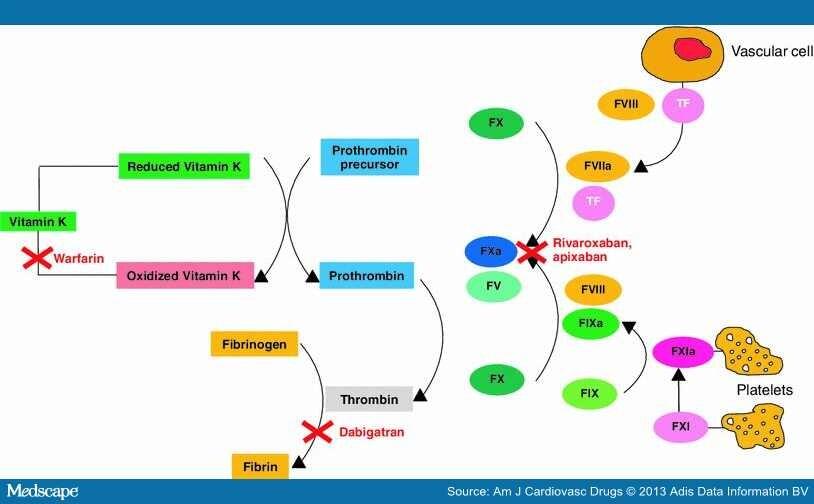
Factor Xa and thrombin as targets for new oral anticoagulants, 2011
New targets
The new oral anticoagulants in the most advanced stages of development are specific and direct inhibitors of either thrombin (Dabigatran) or factor Xa (Rivaroxaban, Apixaban). Although much has been written about the benefits of one target over another, there is ample evidence supporting both thrombin and factor Xa as suitable targets for new anticoagulants. Thrombin is a logical choice because it is the final effector in blood coagulation. Thrombin not only converts fibrinogen to fibrin, but also activates platelets and amplifies its own generation by feedback activation of factors V, VIII and XI. Therefore, thrombin inhibition not only attenuates fibrin formation, but also reduces thrombin generation and may limit platelet activation.
Focusing on Apixaban: Pharmacodynamics and pharmacokinetics
Apixaban is an oral, potent, reversible, direct, and highly selective inhibitor of both free and prothrombinase-bound FXa. It inhibits free human FXa and prothrombinase-bound FXa independently of antithrombin III. (4)
- Apixaban is metabolized by oxidative metabolism via hepatic CYP3A4/5 (as Rivaroxaban, rather than Dabigatran that is not metabolized by cyt P450 isoenzymes but conjugated to acylglucuronides).
- Ketoconazole and ritonavir are examples of strong inhibitors of CYP3A4 and will increase the exposure, whereas rifampicin,
- carbamazepine, and phenobarbital are examples of strong inducers of CYP3A4/5 and will decrease the exposure.
- Apixaban is also a substrate for P-glycoprotein (P-gp), creating an additional factor for drug interactions.
When apixaban is coadministered with drugs that are strong dual inhibitors of CYP3A4 and P-gp, the recommended dose is 2.5 mg twice daily. Food does not affect the bioavailability of apixaban (in reverse it provokes a delayed absorption with Dabigatran and Rivaroxaban).
Approximately 25% of an orally administered apixaban dose is recovered in urine and feces as metabolites. Renal excretion accounts for about 27% of total clearance. That’s why between the important criteria for selecting NOACs generally there is a normal renal function or mild renal dysfunction.

The safety and efficacy of apixaban : where do we stand in 2013?, 2013
Clinical indications & safety / efficacy
-Stroke prevention in AF patients. Apixaban has been evaluated for stroke prevention in patients with nonvalvular AF in 2 large Phase III clinical trials. The AVERROES (Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trial, where the new drug resulted in a significant decrease in stroke or systemic embolism and the rates of major bleeding were similar with apixaban and aspirin. On the other hand, in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial it was compared with dose-adjusted Warfarin; apixaban was clearly superior to warfarin for preventing stroke orsystemic embolism, causing less intracranial bleeding and resulting in lower mortality.
-VTE prophylaxis in major orthopedic surgery. Four clinical trials demonstrated the efficacy and safety of apixaban compared with enoxaparin for the prevention of VTE after major orthopedic surgery. Apixaban was superior to enoxaparin 40 mg once daily and similar to 30 mg twice daily for VTE prevention. This dose of apixaban showed less bleeding than enoxaparin 30 mg twice daily and similar bleeding when compared with enoxaparin 40 mg once daily.
-VTE prophylaxis in acute medical illness. Results of trials demonstrated that an extended course (30 days) of apixaban was not superior to a shorter (6 days) course of enoxaparin.
-Treatment of VTE. Apixaban at either a treatment dose of 5 mf twice daily or a prophylactic dose of 2,5 mg twice daily reduced the risk of recurrent VTE with less risk of major bleeding compared with placebo.
Factor Xa and thrombin as targets for new oral anticoagulants, 2011
Which anticoagulation predictor?
The predictable PK/PD of all NOACs does not necessitate routine monitoring; however, the ability to accurately measure their efficacy is highly desirable in a variety of scenarios including assessment of compliance, and identifying patients at risk for overanticoagulation/bleeding or lack of efficacy.
For Apixaban (and Rivaroxaban), the anti-FXa activity assay is a direct measure of anticoagulation intensity, and thus, the most sensitive and consistent assay for quantitating plasma concentrations.
Apixaban concentration was correlated with INR, PT, and aPTT with less sensitivity.
It is important to keep in mind that the sensitivity and precision of different reagents and instruments used for these coagulation assays is yet to be established. Anyway, quantifying the plasma concentration is likely the most reliable assessment of response and bleeding risk.
Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban, 2013
www.drugs.co. (official FDA information).
When? Decision trees for selecting the right OAC based on individual patient characteristics
Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban, 2013
Selection of Warfarin vs NOAC in AF patients
If we have to choose between a treatment with warfarin or with NOACs, we have to consider the previous treatment:
-if it's based on warfarine, and it's well managed: he has to continue with the same treatment; if not, he has to switch to a NOACs-based treatment.
-if he's not following a treatment with warfarin, but he has a good compliance, we have to start with this kind of treatment.
Indeed, if he has a poor compliance, start with a NOACs one.
We also have to consider the OAC/insurance policy, the access to the INR monitoring and the pacient preference before choosing the treatment .
Selection of appropriate NOAC for AF patients
Because NOACs are dependent on renal excretion, estimated creatinine clearance (CrCl) should be determined before starting treatment with NOACs in all patients. Patients with CrCl less than 30 mL/min are not suitable for NOACs, which means that Warfarin remains still the first choice.
For people with renal impairment we have to differenciate beetween:
-MILD (CrCl 51-80ml/min): Dabigatran (>75y) Apixaban (>80y);
-MODERATE (CrCl 30-50 ml/min): Apixaban is still preferred but also Dabigatran and Rivaroxaban are indicated.
The selection of the adecuate NOAC, also depends from the risk of GI bleed.
-If history of an high risk of GI bleed: patient should take Apixaban, 5 mg (2,5 mg if over 80 or <60 kg )
-if not:
1)Rivaroxaban 20 mg for patient with a poor compliance, or Apixaban, 5 mg (2,5 mg over 80 or >60 kg)
2)poor liver function: Warfarin
3)moderate impairment of CYP3A4: Dabigatran 150 mg (110 mg if over 75)
Converting from or to Apixaban
Switching from warfarin to Apixaban: Warfarin should be discontinued and Eliquis started when the international normalized ratio (INR) is below 2,0.
Switching between Apixaban and anticoagulants other than warfarin: Discontinue one being taken and begin the other at the next scheduled dose. www.drugs.co. (official FDA information).
Dosing adjustments
In some cases we must adjust doses because of the Apixaban’s pharmacokinetic. Renal function is an important variable that must be considered carefully; if the renal impairment is severe we have to reduce the normal dosing of 5 mg twice daily to 2,5 mg. Exactly the same must be done in case of demographic variables, that means halving dosing in patients older than 75-80 years or less than 50 kg. Talking about drug-drug interactions, we should give 2,5 mg when there is coadministration of P-gp inhibitors or CYP 3 A 4 inhibitors (such as Amiodarone, Cimetidine, Ketoconazole, Ritonavir, Verapamil).
No changes are necessary in case of hepatic impairment. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban, 2013
Limits
Apixaban has no established antidote. Recently, modified plasma-derived (pd-FXa) and recombinant FXa (r-FXa) were investigated as potential antidotes for FXa inhibitors.
Given the relatively short half-life of apixaban, it is unclear whether an antidote is really necessary or will result in improved outcomes in patients treated with apixaban. However, immediate reversal of the anticoagulant effect may be needed in cases of major bleeding or emergency procedures. The prothrombin complex concentrate (PCC) could have a role in the reversibility of the anticoagulant effect in patients treated with Apixaban who present with serious bleeding, but this has yet to be proven.
The second major limitation is the lack of standardized coagulation tests for monitoring anticoagulation response, as we already know. Accordingly, directly measuring plasma drug concentrations might be accurate for monitoring anticoagulation. An important consideration for measuring either coagulation or drug exposure is the standardization of sampling time from the last dose. The trough concentration (Ctrough) is preferred over the peak concentration (Cmax), avoiding misinterpretation of results because of variability in the absorption phase.
Finally, safety and efficacy of Eliquis has not been studied in patients with prosthetic heart valves. Therefore, use of Eliquis is not recommended in these patients. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban, 2013
Controindications, Warnings and Precautions
Apixaban is contraindicated in patients with the following conditions:
-Active pathological bleeding
-Severe hypersensitivity reaction to Apixaban.
Discontinuing Apixaban in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from Apixaban to warfarin in clinical trials in patients with nonvalvular atrial fibrillation. If Apixaban must be discontinued for a reason other than pathological bleeding, consider coverage with another anticoagulant.
Eliquis increases the risk of bleeding and can cause serious, potentially fatal, bleeding. Concomitant use of drugs affecting hemostasis increases the risk of bleeding. There isn’t a specific antidote for Apixaban, that means no established way to reverse the anticoagulant effect of this drug (which can be expected to persist for about 24 hours after the last dose, i.e., for about two half-lives). Because of high plasma protein binding, apixaban is not expected to be dialyzable; protamine sulfate and vitamin K would not be expected to affect the anticoagulant activity of apixaban. There is no experience with antifibrinolytic agents (tranexamic acid, aminocaproic acid) in individuals receiving apixabannor experience with systemic hemostatics (desmopressin and aprotinin) in individuals receiving apixaban. www.drugs.co. (official FDA information).
SARA PALACIO E FERDINANDO TOSTO