Introduction

Cobalt is found in many living organisms, including humans.
A cobalt content from 0.13 to 0.30 parts per million in the soil significantly improves the health of farm animals: mammals need small amounts of cobalt salts in the diet.
Cobalt is a key element in vitamin B12.
Furthermore, the metallic cobalt powder may ignite spontaneously in air.
The Co-60, radioactive, is a powerful gamma ray emitter, so the exposure to it increases the risk of cancer.
Ingested, it is only removed from tissues slowly.
It can be produced from nickel and from stable isotopes of cobalt due to neutron irradiation within the nuclear reactor and in case of use of nuclear weapons for neutrons emitted by these.
There are (in theory) nuclear weapons specially designed to increase the amount of 60-Co dispersed in the environment through the fall-out.
High blood levels of cobalt have been associated with neurological alterations such as hand tremor, lack of coordination, cognitive decline, depression, dizziness and loss of hearing and visual capabilities and the best known cardiac effects (arrhythmias and cardiomyopathies), endocrine or allergic.
Cases of Cobaltism concern situations of occupational exposure, past iatrogenic exposure and ion release from metal implants.
The study and the investigation of these cases has revealed a toxicological picture of cobalt probably due to its ability to induce oxidative stress and mitochondrial alterations.
The inhalation of dust during the production of cobalt compounds and the processing of hard metals are the major source of exposure in the workplace.
The main toxic effects, especially those affecting the respiratory system (asthma and pulmonary fibrosis), are given only by cobalt and by association with tungsten carbide which increases pulmonary absorption and the genotoxic potential.
Other cases of neurotoxicity concern intoxication with high doses of cobalt after the intake of cobalt chloride (CoCl2) in anti-anemic therapies. Cobalt
Exposure and effects of cobalt (Co) have been described in many studies, reviews, and toxicology books.
Some of these showed that the main acute and chronic effects concern the respiratory system, heart and thyroid.
Co is classified as group 2A (probably carcinogenic to humans).
Isolated case reports of neurological symptoms after exposure to high doses of Co were also described, following professional exposure, therapeutic ingestion of cobalt chloride (CoCl2) and metallic ion release from cobalt–chromium (Co-Cr) alloy in orthopaedic implants (prosthesis).
Neurotoxicity of cobalt, 2011
Neurotoxicity: mechanisms of action
Effetti neurotossici del cobalto: una questione aperta, 2011
Cobalt occupational or iatrogenic intoxication produces toxic effects on the central and peripheral nervous system, as those found in Cobaltism from arthroplasty: cobalt is directly neurotoxic and epileptogenic.
Cobalt intoxication induces depletion of dopamine, norepinephrine and serotonin.
A particular toxic effect is on the retina and the optic nerve, where you can observe alterations similar to those caused by quinine, tobacco, lead and methanol.
In the pathogenesis of cobalt optic neuropathy the alteration of mitochondrial oxidative phosphorylation plays a fundamental role , since the cobalt induces the loss of mitochondrial membrane potential and the release of apoptogenic factors.

Moreover, cobalt induces the formation of reactive oxygen species (ROS), decreased levels of reduced glutathione and activates the shunt of hexose monophosphate.
A study of Adachi (Effect of hypoxia mimetic cobalt chloride on the expression of extracellularsuperoxide dismutase in retinal pericytes, 2011) also showed that the cobalt chloride contributes to the decrease of extracellular superoxide dismutase in retinal pericytes resulting in increased production of ROS, caspase activation and DNA fragmentation as proapoptotic signals.
Another target of cobalt are the auditory sensory cells, for which oxidative stress induced by the substance causes effects similar to those induced by other ototoxic substances (antimalarials, aminoglycosides, compounds of derivation of the platinum).
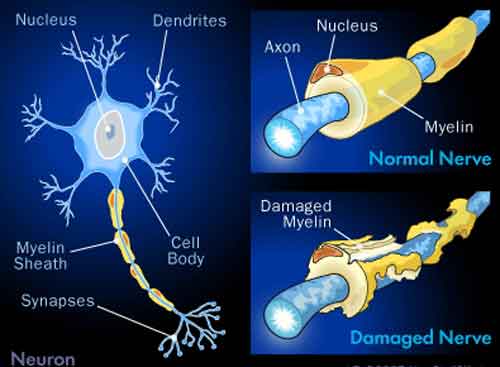
The neurotoxicity is both demyelination that axonal loss, both central and peripheral: the role of ROS in this process is shown, as well as the mechanism of action.
Demyelination: the role of reactive oxygen and nitrogen species, 1999.
The cell death response to the ROS inducer, cobalt chloride, in neuroblastoma cell lines according to p53 status, 2011.
Conclusions
Effetti neurotossici del cobalto: una questione aperta, 2011
The low number of reports of the neurotoxic effects of cobalt may be due related to the nature of the effects attributed to the normal processes of aging or stress.
For this reason it is difficult or even impossible to determine a threshold value because the reported cases relate to high doses and serious and detected effects such as nerve atrophy and total deafness.
However at the same conditions, the appearance and severity of neurological symptoms seem to depend on individual susceptibility such as the predisposition to cobalt-induced mitochondrial alterations and the binding of cobalt with albumin: due to diseases like trauma, diabetes, infections, renal failure, the binding site of the cobalt with albumin may change, which results in an increased level of circulating free cobalt responsible for the toxicity.
The series of symptoms that emerge from the studies examined include hypothyroidism, polycythemia, cardiomyopathies, and pulmonary complications (especially when cobalt is inhaled), impaired vision and hearing, dizziness, dysesthesia, tremor, fatigue, depression, irritability and memory loss.
It seems that the retinal ganglion cells are the target of the action of cobalt, as their vulnerability to oxidative damage and mitochondrial alterations leads to confirm the mechanisms of action mentioned above.
Luca Calorio, Valeria Farina e Alberto Cavaliere