STRUCTURE

Lipoic acid metabolism and mitochondrial redox regulation, 2017
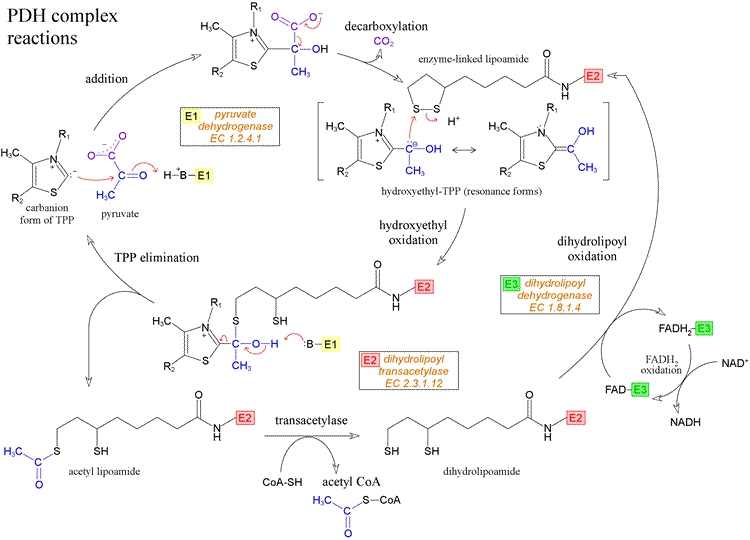
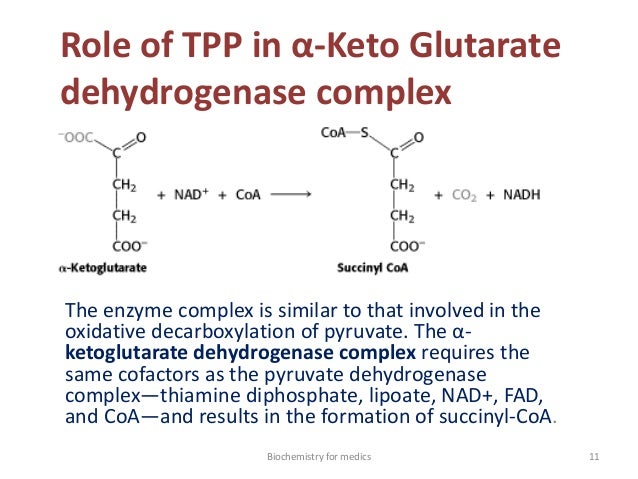
- Lipoic acid is an essential cofactor for mitochondrial metabolism and is synthesized de novo using intermediates from mitochondrial fatty-acid synthesis type II, S-adenosylmethionine and iron–sulfur clusters. This cofactor is required for catalysis by multiple mitochondrial 2-ketoacid dehydrogenase complexes, including pyruvate dehydrogenase, -ketoglutarate dehydrogenase, and branched-chain ketoacid dehydrogenase.

Lipoic acid also plays a critical role in stabilizing and regulating these multienzyme complexes. Many of these dehydrogenases are regulated by reactive oxygen species, mediated through the disulfide bond of the prosthetic lipoyl moiety. Collectively, its functions explain why lipoic acid is required for cell growth, mitochondrial activity, and coordination of fuel metabolism.

The lipoic acid (LA) cofactor is covalently bound to protein via an amide bond (lipoamide) to the ε-amino group of a lysine in a conserved lipoylation domain. The oxidized form is ready for reaction with different substrates and forms an intermediate until the substrate is released and the reduced form with two sulfhydryls (dihydrolipoamide) is generated. DLD catalyzes the reactivation of the cofactor by a redox reaction with nicotinamide adenine dinucleotide (NAD + )
METABOLISM
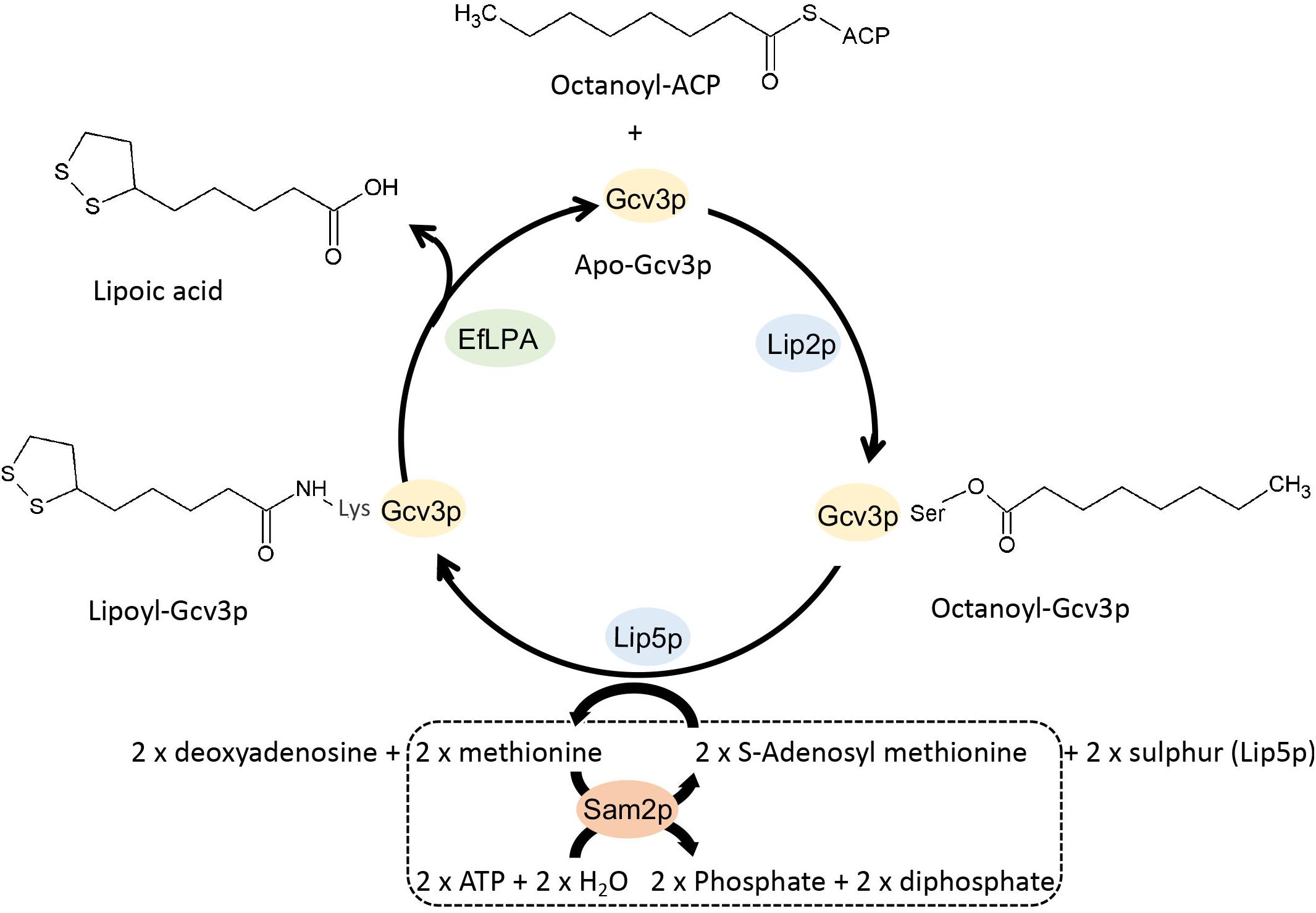
Synthesis requires Gcv3p-octanoic acid plus 2 x S-deoxyadenosylmethionine
Mechanism-Driven Metabolic Engineering for Bio-Based Production of Free R-Lipoic Acid in Saccharomyces cerevisiae Mitochondria, 2020


Nebraska
Targeting mitochondrial oxidative stress through lipoic acid synthase: a novel strategy to manage diabetic cardiovascular disease.2012
Lipoic acid (LA) is a potent mitochondrial antioxidant and an essential cofactor of α-ketoacid dehydrogenases.

Erythropoiesis and iron sulfur cluster biogenesis., 2010
ISCA and C1orf69 are thought to assemble Fe-S clusters for mitochondrial aconitase and for lipoate synthase, the enzyme producing lipoate for pyruvate dehydrogenase complex (PDC). PDC and aconitase are involved in the production of succinyl-CoA, a substrate for heme biosynthesis. Thus, many steps of heme synthesis depend on Fe-S cluster assembly.
TRANSPORT

[Click box to select records for export to bibliographic management software or to a spreadsheet.]
Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. 2007
In most cases (with the exception of the sodium-dependent multivitamin transporter for biotin, pantothenic acid, and lipoic acid), the vitamins are transported by separate carriers through the blood-brain barrier or choroid plexus.
Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. 2015
Sodium dependent multivitamin transporter (SMVT; product of the SLC5A6 gene) is an important transmembrane protein responsible for translocation of vitamins and other essential cofactors such as biotin, pantothenic acid and lipoic acid.
FUNCTION
LA is covalently bound to the ε-amino group of lysine residues and functions as a cofactor for mitochondrial enzymes by catalyzing the oxidative decarboxylation of pyruvate, α-ketoglutarate and branched-chain α-keto acids. LA and its reduced form - dihydrolipoic acid (DHLA), meet all the criteria for an ideal antioxidant because they can easily quench radicals, can chelate metals, have an amphiphlic character and they do not exhibit any serious side effects. They interact with other antioxidants and can regenerate them. For this reason, LA is called an antioxidant of antioxidants. LA has an influence on the second messenger nuclear factor κB (NF-κB) and attenuates the release of free radicals and cytotoxic cytokines. Lipoic acid - biological activity and therapeutic potential., 2011




GSH and Thioredoxin??
The role of R-α-lipoic acid as a cofactor (lipoyllysine) in mitochondrial energy metabolism is well established. Lipoic acid non-covalently bound and exogenously administered to cells or supplemented in the diet is a potent modulator of the cell's redox status. The diversity of beneficial effects of lipoic acid in a variety of tissues can be mechanistically viewed in terms of thiol/disulfide exchange reactions that modulate the environment's redox and energy status. Lipoic acid-driven thiol/disulfide exchange reactions appear critical for the modulation of proteins involved in cell signaling and transcription factors. This review emphasizes the effects of lipoic acid on PI3K and AMPK signaling and related transcriptional pathways that are integrated by PGC-1α, a critical regulator of energy homoestasis. The effects of lipoic acid on the neuronal energy-redox axis are largely reviewed in terms of their outcomes for aging and age-related neurodegenerative diseases.
Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. 2011
Cofactor of PDH

Cofactor of alphaKGDH
Redox cycling

Bone metabolism
Emerging role of alpha-lipoic acid in the prevention and treatment of bone loss, 2015

Lipoic acid metabolism and mitochondrial redox regulation, 2018