MicroRNAs are post-transcriptional regulators that bind to complementary sequences in the three prime untranslated regions (3' UTRs) of target messenger RNA transcripts (mRNAs), usually resulting in gene silencing.

more details
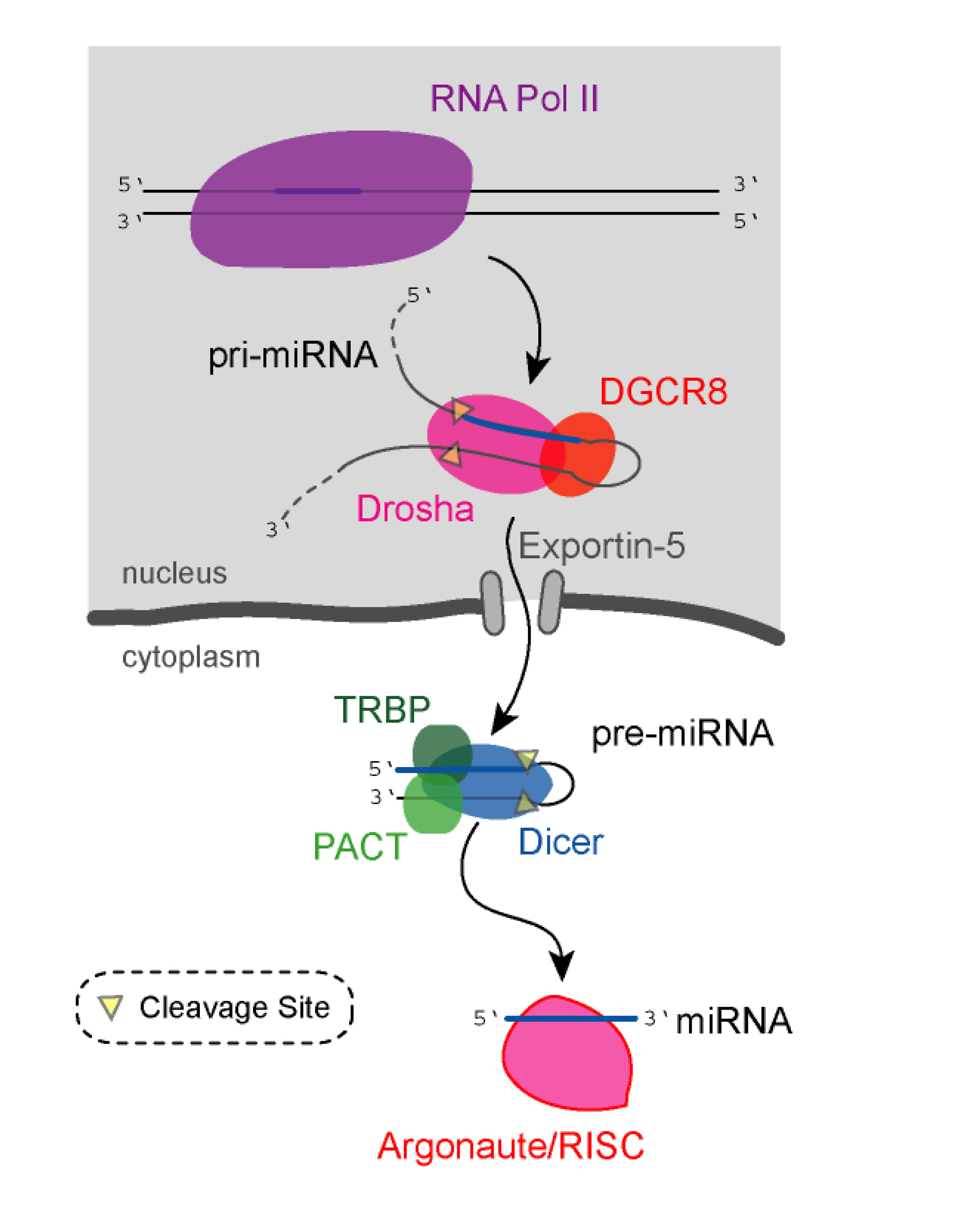
Nuclear Enzymes


RNP and XPO5
- higher Methionine (more mTOR?)
Cytoplasmic Enzymes


Argonaute environment
Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia. 2010
Prolyl 4-hydroxylase. 2010
miRNA genes and the brain: implications for psychiatric disorders. 2010

Genome-wide transcriptional profiling reveals microRNA-correlated genes and biological processes in human lymphoblastoid cell lines. 2009
To examine miRNA regulatory effect on global gene expression under endogenous condition, we performed pair-wise correlation coefficient analysis on expression levels of 366 miRNAs and 14,174 messenger RNAs (mRNAs) in 90 immortalized lymphoblastoid cell lines, and observed significant correlations between the two species of RNA transcripts. We identified a total of 7,207 significantly correlated miRNA-mRNA pairs (false discovery rate q<0.01). Of those, 4,085 pairs showed positive correlations while 3,122 pairs showed negative correlations. Gene ontology analyses on the miRNA-correlated genes revealed significant enrichments in several biological processes related to cell cycle, cell communication and signal transduction. Individually, each of three miRNAs (miR-331, -98 and -33b) demonstrated significant correlation with the genes in cell cycle-related biological processes, which is consistent with important role of miRNAs in cell cycle regulation.
Aging and microRNA
Epigenetic Control of MicroRNA Expression and Aging, 2009