Davide Cussa
Francesco Giuseppe Ecclesia
"Puberty begins with a kiss."
Definition
Kisspeptins, encoded by the Kiss1 gene, and their canonical receptor, GPR54 (also termed Kiss1R), are unanimously recognised as essential regulators of puberty onset and gonadotrophin secretion.
Formerly known as Metastin, is a G-protein coupled receptor ligand for GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis.
Genomics
Kisspeptin (KP) is a peptide that acts as a ligand to the KISS1 receptor (formerly GPR54). Specific areas of the brain that express KP are the arcuate nucleus, periventricular nucleus and anteroventral periventricular nucleus, which are all nuclei of the hypothalamus. Additionally, it has been found that 75% of all GnRH neurons co-express KISS1. When kisspeptin binds to KISS1, it initiates a G-protein signaling cascade that ultimately drives the production and release of GnRH.
This gene is located on the long arm of chromosome 1 (1q32) and has four exons of which the 5' and 3' exons are only partly translated. The gene product is a 145 amino acid precursor peptide which is cleaved to 54 amino acids in length, which may be further truncated to 14, 13 or 10 amino acid carboxyl terminal fragments. These N-terminally truncated peptides are known as the kisspeptins and belong to a larger family of peptides known as RFamides which all share a common arginine-phenylalanine-NH2 motif at their C-terminus.
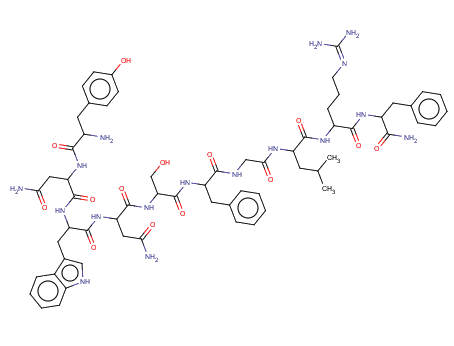
Structure
Hydrogen bonds are important within these peptides, with eight hydrogen bonds being formed within Kisspeptin-13, allowing 16 hydrogen constraints. These are formed between residues Phe9, Leu11, Arg12 and Phe13. These four residues have also been shown to form a pharmacophore which is important for receptor binding. All kisspeptins fragments have also been shown to contain a matrix metalloproteinase (MMP) mediated cleavage site, which is thought to be a mechanism to inactivate the protein. This site is at the C-terminus consisting of Phe-Gly-Leu-Arg, were MMP-2 and MMP-9 can cleave kisspeptin-54 between Gly51 and Leu52. MMPs have also been shown to form a complex with the kisspeptin pre-peptide involving the N-terminal 48 amino acids with Cys53 being critical to this process and the propeptide domain of MMPs.
Cellular localization and functions
As we already mentioned in the previous part, Kisspeptin is expressed abundantly in the arcuate nucleus (Arc) and the anteroventral periventricular nucleus (AVPV) of the forebrain. We also know that the gene Kiss1 is transcribed in the vascular endothelium and in pancreas within the islet cells. It also has an important function in these sites. We know, according to more and more studies, that Kisspeptin-GPR54 signaling is essential to initiate gonadotropin (LH/FSH) secretion at puberty.
Kisspeptin neurons may act as central processors for relaying signals from the periphery to GnRH neurons. Metabolic and environmental factors regulate reproductive function, which ensures that reproduction proceeds only when metabolic and environmental conditions are favorable. Kisspeptin stimulates GnRH secretion, and Kiss1 mRNA is both negatively and positively regulated by sex steroids. The expression of Kiss1 may be induced by leptin, whose plasma levels reflect the state of metabolic reserves. Kisspeptin neurons may also receive input from the hypothalamic-pituitary-adrenal axis and from environmental cues such as photoperiod via the suprachiasmatic nucleus of the hypothalamus (SCN).
Kisspeptin initiation of Hypothalamic-Pituitary-Gonadal Axis
Studies in several mammalian species have shown that kisspeptins stimulate the secretion of gonadotropins from the pituitary by stimulating the release of GnRH from the forebrain after the activation of GPR54, which is expressed by GnRH neurons.
Kisspeptin stimulates the neuroendocrine reproductive axis, and sex steroids differentially regulate the expression of Kiss1 mRNA in different nuclei within the forebrain. Kisspeptin released by neurons in the AVPV and Arc stimulates GnRH release, which induces the release of LH and FSH. The gonads respond to gonadotropins by secreting sex steroids, which then feed back to regulate the activity of kisspeptin neurons, inhibiting Kiss1 expression in the Arc and inducing its expression in the AVPV. The inductive effect of sex steroids on Kiss1 expression in the AVPV may contribute to the preovulatory LH surge in females (and possibly T-mediated sex behavior in the male).
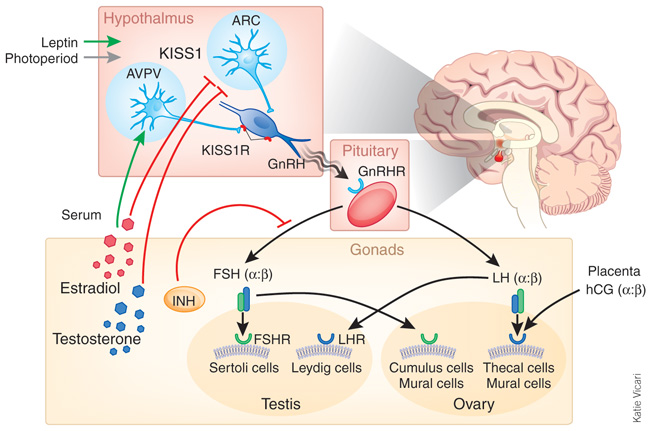
Both estradiol and testosterone regulate the expression of the Kiss1 gene in the Arc and AVPV; however, the response of the Kiss1 gene to these steroids is exactly opposite between these two nuclei. Estradiol and testosterone down-regulate Kiss1 mRNA in the Arc and up-regulate its expression in the AVPV. Thus, kisspeptin neurons in the Arc may participate in the negative feedback regulation of gonadotropin secretion, whereas kisspeptin neurons in the AVPV may contribute to generating the preovulatory gonadotropin surge in the female.
Sex steroids also regulate the expression of co-transmitters of Kiss1 neurones, such as neurokinin B, whose mRNA content in the ARC fluctuates in parallel to that of Kiss1 in response to changes in the circulating levels of sex steroids, therefore suggesting the contribution of this neuropeptide in the feedback control of gonadotrophin secretion.
Hypothalamic levels of Kiss1 and GPR54 mRNA increase dramatically at puberty, suggesting that kisspeptin signaling could mediate the neuroendocrine events that trigger the onset of puberty. Together, these observations demonstrate that kisspeptin-GPR54 signaling in the brain serves as an important conduit for controlling GnRH secretion in the developing and adult animal.
Cellular action of kisspeptins on GnRH neurons
Kisspeptin signaling through GPR54/KISS1R couples to Gq/11 to activate phospholipase C and increases inositol triphosphate (IP3) and diacylglycerol (DAG) levels in the cell. The subsequent increase in intracellular Ca2+ and DAG activates protein kinase C and initiates a kinase phosphorylation cascade resulting in phosphorylation of ERK1/2. DAG also stimulates GnRH depolarization by activation of a nonselective cation channel (TRPC) and inhibition of an inwardly rectifying potassium channel (Kir). Thus kisspeptins act directly on GnRH neurons to induce a sustained depolarization event and increase the rate of action potential firing. The number of GnRH neurons that can respond to kisspeptins increases during puberty even though the expression levels only change slightly, suggesting posttranslational maturation of GPR54/KISS1R function with puberty. In adult mice, γ-amino-butyric acid (GABA) has a predominantly hyperpolarizing effect on GnRH neurons to inhibit GnRH release. Kisspeptins have been reported to suppress the inhibitory effects of GABA receptor signaling in GnRH neurons. Thus, under conditions of estrogen positive feedback when kisspeptin levels increase in the AVPV, kisspeptins will antagonize any inhibitory action of GABA to stimulate GnRH release and LH surge/ovulation.

GPR54 and its role in reproductive disorders
The kisspeptin receptor (KISS1R) is a G protein-coupled receptor recognized as the trigger of puberty and a regulator of reproductive competence in adulthood . Inactivating mutations in KISS1R identified in patients have been associated with iodiopathic hypogonadotropic hypogonadism (IHH) and precocious puberty.
IHH is characterised by abnormal/absent GnRH-induced luteinising hormone (LH) pulsations in which patients present with delayed or absent pubertal development and other associated somatic abnormalities. Although rare, IHH is a disease caused by defects in the synthesis, secretion and action of GnRH. Loss-of-function mutations in GPR54 cause the normosmic form of hypogonadotrophic hypogonadism. It now appears that the opposite is also true: a gain-of-function mutation in GPR54 appears to lead to central precocious puberty.
Kisspeptin and GPR54: Discovery of a Novel Pathway in Reproduction
The Role of Kisspeptin Signaling in Reproduction