Parathyroid Hormone-Related Protein (or PTHrP) is a protein member of the parathyroid hormone family.
PTHrP was initially discovered as a protein secreted by certain tumors that caused hypercalcemia in affected patiens, by stimulatin resorption of calcium from bone and suppressing calcium loss in urine (similar activities of the regular Parathyroid Hormones). However it was soon shown that PTHrP had many effects not seen with parathyroid hormone.
Hormone structure, Receptors and Sources
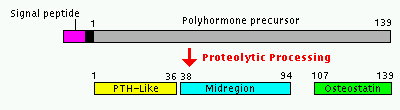
PTHrP is a 139- to 173-amino-acid protein with N-terminal homology to parathyroid hormone (PTH). It should probably be described as a polyhormone, because a family of peptide hormones are generated by alternative splicing of the primary transcript and through use of alternative post-translational cleavage sites.
The figure shows one of the characterized processing patterns of the PTHrP preprohormone,
resulting in three bioactive peptides: the N-terminal homologous to PTH, the midregion PTHrP that augments calcium transport and the C-terminal peptide, Osteostatin, that is a potent inhibitor of bone resorption.
It can be useful to observe the protein aminoacids percentage of preproPTH and preproPTHrP

The different activities of PTHrP result also from use of multiple receptors: the amino-terminal peptides share a receptor with paratyroid hormone, but they also bind to other type of receptors. Moreover, it is clear that the midregion and osteostatin peptides bind unique receptors. Using these receptors PTHrP acts an endocrine, and especially, autocrine and paracrine hormone.
PTHrP acts also as an intracrine hormone: a form of PTHrP is not secreted and, via nuclear targeting sequences, is traslocated to the nucleus, where it affects nuclear function (such as controlling programmed cell death).
Its gene is expressed in different set of tissues, and during both fetal and postnatal life: several types of epitelium, mesenchyme, vascular smooth muscle and central nervous system are known to secrete PTHrP.
Some of the effects of PTHrP result on transepithelial fluxes of calcium, as his name suggest; however many of its actions regulate the proliferation, differentiation and death of many cell types.
Parathyroid hormone-related protein: structure, function, and measurement,1992.
PTHrP in Cartilage and Bone Development
In fetal bone, PTHrP is synthesized exclusively by perichondrial cells and chondrocytes at the ends of the growing bones, then diffuses away from the sites of production and binds to PTH/PTHrP receptors on nearby chondrocytes. PTHrP acts on them to keep the chondrocytes proliferating and delay their differentiation into prehypertrophic and then hypertrophic chondrocytes. PTHrP also increases the rate of chondrocyte’s proliferation. After chondrocytes stop proliferating, they synthesize Indian Hedgehog (Ihh). In a feedback loop, Ihh increases the synthesis of PTHrP, to accelerate the differentiation of round proliferative chondrocytes into flat proliferating chondrocytes, to increase the rate of proliferation of adjacent chondrocytes, and to direct perichondrial cells to differentiate into osteoblasts.

Activation of the PTH/PTHrP receptor leads to activation of multiple G proteins. The activation of Gs leads to subsequent activation of adenylate cyclase and generation of cyclic AMP. Cyclic AMP has several actions, including the activation of protein kinase A (PKA). PKA then leads to decreases in both p57 levels and in Runx2 mRNA and protein. p57 is a member of the CIP/KIP family of inhibitors of cyclin-dependent kinases; decrease of p57 leads to increase proliferation and delayed differentiation of chondrocytes. The transcription factor Runx2 is required for the hypertrophic differentiation of fetal chondrocytes in most bones: the suppression of Runx2 production by PTHrP probably contributes to the delay differentiation of chondrocytes. PKA also phosphorylates SOX9, increasing its activity.

Human fetuses with defective or absent PTH/PTHrP receptors ( Blomstrand chondro-osteodystrophy ) die in utero with skeletal abnormalities; further, humans with Jansen chondro-osteodystrophy manifest growth abnormalities caused by the PTH/PTHrP receptor constitutively active.
How PTHrP controls growth plate chondrocytes,2005.
PTHrP and Cancer
PTHrP was discovered as a protein secreted by certain tumors, especially carcinomas of breast, lung (squamous), head and neck (squamous), kidney, bladder, cervix, uterus, and ovary. In fact circulating levels in serum are very low in physiological conditions, but they become relevant when there is extreme and sustained over-production of PTHrP. This uncontrolled secretion of PTHrP causes a relevant cross-talk with PTH/PTHrP receptor and influences plasmatic concentration of calcium, contributing to the appearance of Hypercalcemia of Malignancy (HMM).
PTHrP mimics PTH action: it stimulates reabsorption of calcium from bone and enhances renal retention.
Test ID: PTHRP
PTHrP has also an influence in development of bone metastasis. Breast and prostate cancers and multiple myeloma most frequently produce bone metastasis. In particular osteolytic metastasis can evolve through a series of tumor-microenvironment interactions known as the ”vicious cycle”: in that cycle PTHrP has an important role. Tumor cells secrete factors that stimulate osteoblast activation of osteoclasts. Activated osteoclasts degrade the bone matrix and release factors that stimulate tumor cells.
Metastatic tumor cells interact with the bone microenvironment to facilitate osteolytic colonization. Tumor cells secrete PTHrP, which stimulates osteoblasts (bone-forming cells) to produce both a membrane-bound RANKL and OPG, a soluble decoy receptor for RANKL. It is the ratio of RANKL to OPG that determines osteoclast (bone-degrading cell) activation, through its receptor for RANKL. Activated osteoclasts degrade the bone matrix, releasing into the local microenvironment embedded growth factors including TGF-ß. TGF-ß stimulates tumor-cell PTHrP production, renewing the cycle.

In this case, plasma level of PTHrP can also be used as a biomarker associated with bone metastasis.
PTHrP and other effects
PTHrP regulates epithelial-mesenchymal interactions during the formation of the mammary glands. This effect was confirmed in female mice with homozygous inactivation of PTHrP gene: the mammary glands fail to develop, except for the earliest stages.
Parathyroid hormone-related protein specifies the mammary mesenchyme and regulates embryonic mammary development,2013.
Elevated plasma levels of PTHrP can be found during lactation because mammary epithelial cells secrete large amounts of PTHrP, which suggests a role of this hormone in adapting maternal metabolism to the calcium demands. The mammary epithelium possesses a local serotonin signaling system which drives PTHrP expression during lactation and in breast cancer cells. The mammary gland serotonin system is highly induced in response to alveolar dilation due to milk secretion.
New concepts of breast cell communication to bone,2014.
PTHrP has profound effects on a large number of physiologic processes, determining a complex system of interaction.
PTHrP is secreted from smooth muscle in many organs, usually in response to streching; it relaxes smooth muscle, serving also as a vasodilating hormone. However it may also have effect on contraction of muscle in the bladder, uterus and heart.
PTHrP/ PTHrP receptor signaling is also involved in tooth development, in placental transfer of calcium and it appears to influence neural survival by several mechanisms.
Osteostatin and Osteoporosis
Osteostatin has an inhibitory effect on bone resorption in vitro and in vivo. It exerts its inhibitory effect in a biphasic manner on TRAcP activity, inhibiting its secretion and either suppressing its synthesis or increasing its degradation. In addition, osteostatin induced rapid cellular retraction of both human and rat cultured osteoclasts, which was morphologically distinct from that produced by calcitonin.
Its role in the regulation of adult bone mass isn't well-known yet; although an osteostatin deficiency may contribute to the pathogenesis of elderly osteoporosis.
A potent inhibitor of osteoclastic bone resorption,1991.
Erica Rosina