INTRODUCTION
The renin-angiotensin-aldosterone pathway is a hormone system which regulates blood pressure and fluid balance, while Anrep effect is an increase of heart contractility in response to a greater afterload, as it appens in Hypertension.
RAAS PATHWAY

Following blood pressure decrease, sympathetic stimulation or NaCl decrease in distal tubule (macula densa), kidney granular cells in the afferent arteriole wall release renin, an enzyme that converts circulating liver-secreted angiotensinogen into angiotensin I; angiotensin-converting-enzyme (ACE), produced by the endothelium and therefore mainly located in lungs, then performs conversion of angiotensin I into angiotensin II. This carries out many effects by activating IP3-PKC pathway: blood vessels constriction, increase of Na/H transporter activity in proximal tubule, slight increase of ADH release and aldosterone secretion by adrenal cortex. Aldosterone promotes Na intake and K excretion in colon and kidney, through ENAC and ROMK channels production and migration toward cell membrane at the luminal side, and Na/K pump toward the basal side. Since this steroidal hormone increases K secretion, its release from adrenal cortex is also stimulated by hyperkalemia. Sodium drags fluids into blood vessels, restoring normal pressure values.

Many disorders involve RAAS dysregulation:
• Primary hyperaldosteronism, is characterized by the overproduction of the mineralocorticoid hormonealdosterone by the adrenal glands
• Secondary hyperaldosteronism refers to an abnormality that indirectly results in pathology through a predictable physiologic pathway. One cause is a juxtaglomerular cell tumor. Another is renal artery stenosis, in which the reduced blood supply across the juxtaglomerular apparatus stimulates the production of renin. Other causes can come from the tubules: Hyporeabsorption of sodium (as seen in Bartter and Gitelman syndromes) will lead to hypovolemia or hypotension, which will activate the RAAS system.
• Pseudohyperaldosteronism is a medical condition that mimics hyperaldosteronism. Like hyperaldosteronism, it produces hypertension associated with low plasma renin activity, and metabolic alkalosis associated with hypokalemia. Unlike hyperaldosteronism, it involves aldosterone levels that are normal or low. Dietary causes include the chronic excessive ingestion of licorice which is rich of glycyrrhizic acid inhibiting 11-β-Hydroxisteroid dehydrogenase; genetic causes include Liddle’s Syndrome.
• Hypoaldosteronism refers to decreased levels of the hormone aldosterone. It may be due to a primary deficiency, as in Addison’s disease, or to a secondary one such as in diabetic nephropaty.
• Pseudohypoaldosteronism is a condition that mimics hypoaldosteronism. However, the condition is due to a failure of response to aldosterone, and levels of this hormone are actually elevated, due to a lack of feedback inhibition. Pseudohypoaldosteronism tipe 1 is a condition characterized by problems regulating the amount of sodium in the body. It is triggered by mutations in ENAC subunits, and it leads to hyperkalemia and metabolic acidosis. On the other hand, Pseudohypoaldosteronism tipe 2 (Gordon’s Syndrome) concerns mutations of two related genes (WNK1 e WNK4) causing ROMK and NCC dysregulation; this eventually leads to a hypertension, hyperkalemia and metabolic acidosis.
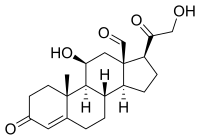
ANREP EFFECT
It has been demonstrated that angiotensin II and maybe aldosterone are implicated in myocardial contractility increase following greater preload or after load.
The Anrep effect: 100 years later, 2013
The heart is under continuous nervous, hormonal, and electrophysiological influence. In spite of this, the cardiac muscle has intrinsic mechanisms to adapt cardiac output to changes in hemodynamic conditions. An increase in left ventricular end-diastolic volume (EDV), caused by either increasing aortic resistance to ejection or venous return, immediately leads to a more powerful contraction. This is the well known Frank-Starling mechanism that allows the heart to increase its output after a rise in preload or to maintain it despite a greater after load.
A role for the sarcolemmal Na+/H+ exchanger in the slow force response to myocardial stretch, 1999
The Anrep effect: an intrinsic myocardial mechanism, 1988
However, after this initial rise in contractility, and over the 10 to 15 minutes following a sudden stretch, myocardial performance continues to increase. In 1912, Gleb Von Anrep showed that after clamping ascending aorta in a dog its end diastolic volume initially increased, keeping systolic ejection as stated by Frank-Starling law, and then decreased, although cardiac output was not affected by this drop. Anrep surmised that this was due to a positive inotropic effect. In 1960, Sarnoff coined the term “homeometric autoregulation” to define, in isolated hearts, the progressive decrease in left ventricular end-diastolic pressure (EDP) that occurs after its initial increase induced by an afterload rise. Further research has cast new light on molecular pathways involved in this mechanism. Greater afterload triggers angiotensin II release from cardiomyocytes through stretch receptors such as integrins. Angiotensin II binds to AT I receptor, then endothelin-1 is released and binds to its own receptor, ETa.
Pubmed, 'The Anrep effect and myocardial hypertrophy', 2005
Mechanisms underlying the increase in force and Ca++ transient that follow stretch of cardiac muscle,1999
This autocrine stimulation seems to enhance production of mineralcorticoid hormones by myocytes (although this point is currently under discussion, since myocardial cells have a very poor set of steroidal-production enzymes), which acts through mineralcorticoid receptor; it has been proposed that EGFR transactivation is also involved.
The Anrep effect requires transactivation of the epidermal grow factor receptor,2010
This pathway boosts up NADPH oxidase activity and supeoxide anion production, leading to mitochondrial K-channels opening, consequent depolarization and further ROS production. Redox-sensitive kinases ERK1/2 and p90 then phosphorylate NHE1, which increases Na amount in cells and thus NCX activity, bringing more Ca into cardiomyocytes. Therefore, this pathway explains the positive inotropic effect following a greater afterload, although other mechanism have been suggested.
The Anrep Effect Reconsidered,1972

CONCLUSIONS
There is enough evidence to suggest that the Anrep effect takes place after a series of events, with the release of Ang II igniting this molecular cascade, and ending with an increase in the Ca transient through activation of the NCX. Interestingly, 50 years after Anrep reported his phenomenon, Sarnoff coined the word "autorregulación", a term transpiring that the mechanism resides in the myocardium itself. A century later, this is quite evident in light of the new cardiac autocrine/paracrine mechanisms described herein.
Alberto Pacielli, Nicola Marchese