Palmitoylethanolamide / PEA is an endogenous fatty acid amide, belonging to the class of nuclear factor agonists. PEA has been demonstrated to bind to a receptor in the cell-nucleus (a nuclear receptor) and exerts a great variety of biological functions related to chronic pain and inflammation. The main target is thought to be the peroxisome proliferator-activated receptor alpha (PPAR-α). PEA also has affinity to cannabinoid-like G-coupled receptors GPR55 and GPR119. PEA cannot strictly be considered a classic endocannabinoid because it lacks affinity for the cannabinoid receptors CB1 and CB2. However, the presence of PEA (and other structurally related N-acylethanolamines) has been known to enhance anandamide activity by an "entourage effect".
Synthesis
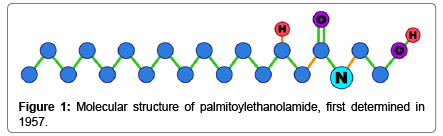

Function
- PEA is an anti-inflammatory and neuroprotective compound.
- Pharmacological tools are available to control PEA levels via FAAH and NAAA inhibition.
- FAAH and NAAA differentially control PEA levels in a tissue/disease-specific manner.
Harnessing the anti-inflammatory potential of palmitoylethanolamide 2014
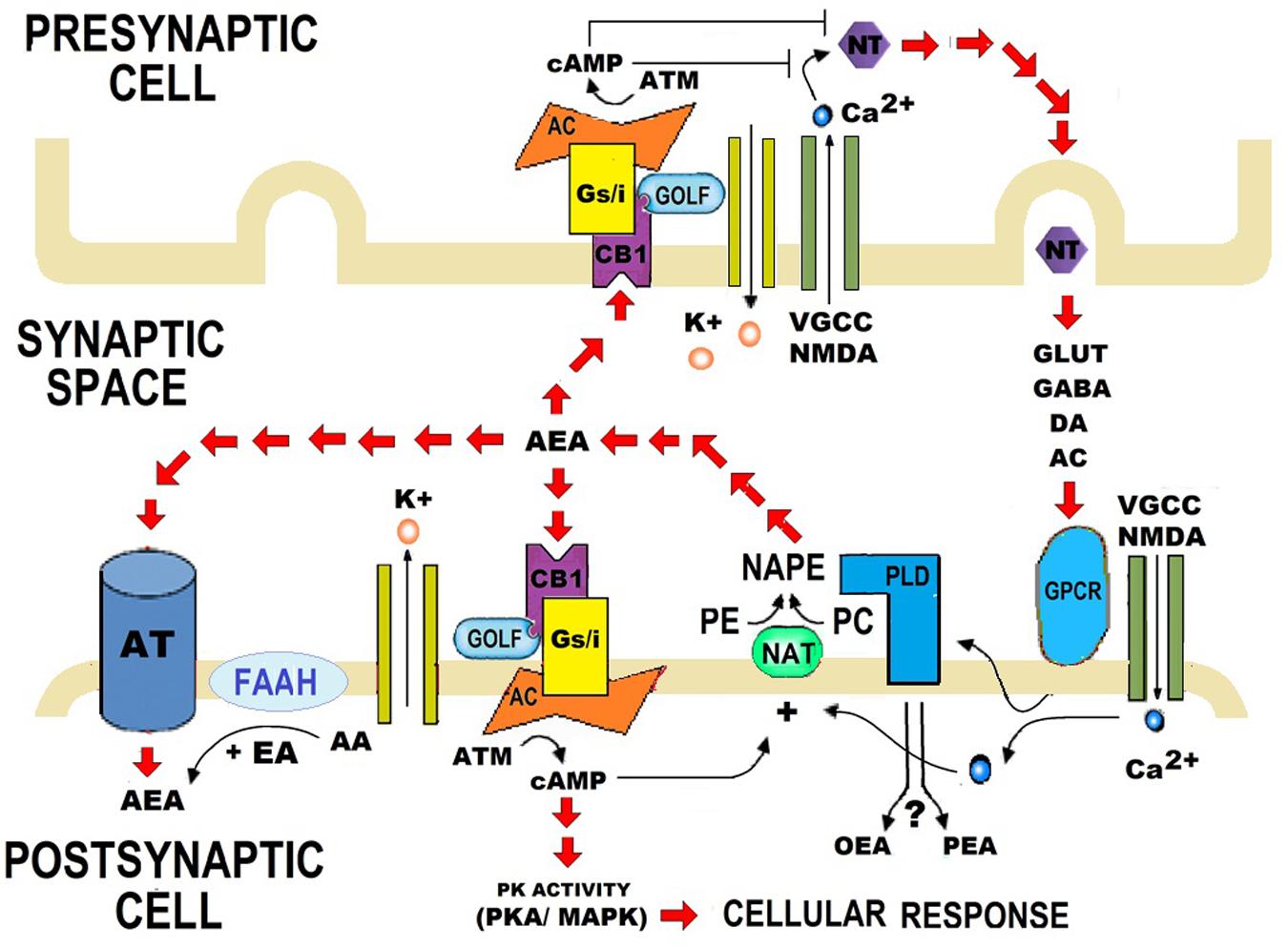
Glial Modulation by N-acylethanolamides in Brain Injury and Neurodegeneration, 2016

Palmitoylethanolamide synthesis and catabolism. A plasma membrane-associated N-acylated phosphatidylethanolaminephospholipase D (PLD) converts N-palmitoylphosphatidyl-ethanolamine (N-APE) into palmitoylethanolamide and phosphatidic acid. Palmitoylethanolamide is metabolized to palmitic acid and ethanolamine by fatty acid amide hydrolase (FAAH, which also breaks down other fatty acid amides) and the more selective N-acyl ethanolamine-hydrolyzing acid amidase (NAAA). Tissue levels of palmitoylethanolamide increase in stressful settings such as peripheral tissue inflammation, neuroinflammation and pain. See text for further details. [Reprinted from: Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology 2014; 141(3): 314-27 (Fig. 2)].