γ-Glutamyl Cycle is mainly a glutathione salvage pathway
.

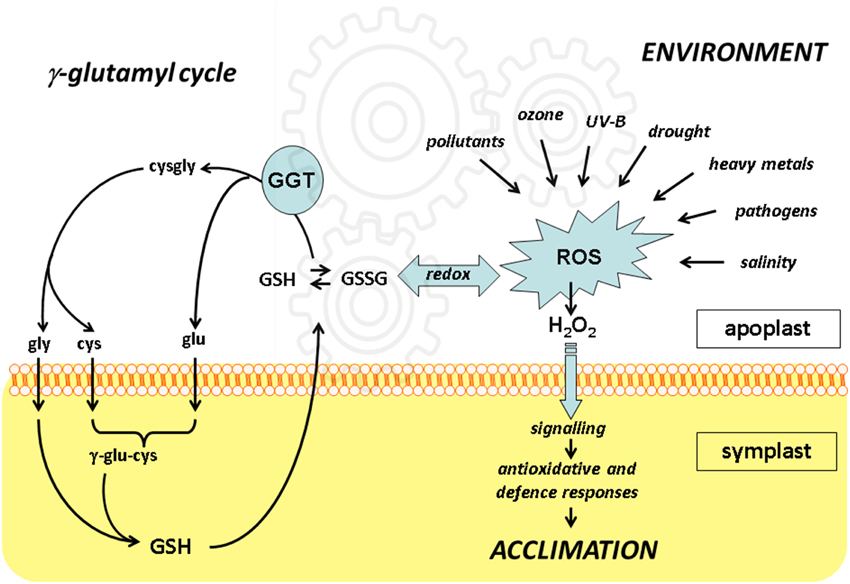
The γ-glutamyl cycle: an essential pathway for the cell to maintain adequate intracellular glutathione (GSH) levels. γ-GT: γ-glutamyltransferase.

The enzymes involved are:
- γ-glutamyltranspeptidase (GGT)
- γ-glutamyl-cyclotransferase
- Catalyzes the formation of 5-oxoproline from gamma-glutamyl dipeptides and may play a significant role in glutathione homeostasis. Induces release of cytochrome c from mitochondria with resultant induction of apoptosis
- 5-oxoprolinase
- glutamate-cysteine ligase (GCL)
- glutathione synthase (GS).

The biosynthesis of glutatione in cells is accomplished by six enzymes found on the plasma membrane and in the cytosol from the precursor amino acids Glu, Cys, and Gly. Extracellular GSH is selectively transported into the cell but somer of tissues requiring degradation to single amino acids before transport into the cell. The universal facilitator of GSH breakdown and only know glutathionase enzyme is γ-glutamyltranspeptidase (GGT) which is located on the outer leaf of the cell's plasma membrane. γ-glutamyltranspeptidase cleaves glutathione at the unique γ-glutamyl bond to form free glutamate and the dipeptide Cys-Gly, which is degraded to Cys and Gly for transport in to cells via amino acid transporters. The Glu is quickly reunited with another amino acid (usually a Cys) and transported into the cell as γ-Glu-amino acid. Once inside the cell, precursor amino acids are enzymatically processed and passed along to enzymes responsible for GSH synthesis beginning with cyclization and decyclization reactions to release the bound amino acid and present free Glu as the first substrate precursor for GSH biosynthesis. Glu and Cys are combined in to form γ-glutamylcysteine using the enzyme glutamate-cysteine ligase (GCL). The last step of GSH biosynthesis involve the addition of Gly by ATP and Mg2+ dependent glutathione synthase (GS).
DEFINITION
A short protein description with the molecular wheight, isoforms, etc...
Use, when available, the link to Wikipedia (Es Trypsin)
External links not available on Wikipedia have to be added here
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage (Width 700 px)


SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,
biological function
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC USE
A γ-Glutamyl Cyclotransferase Protects Arabidopsis Plants from Heavy Metal Toxicity by Recycling Glutamate to Maintain Glutathione Homeostasis, 2013
γ‐Glutamyl Transpeptidase in Glutathione Biosynthesis, 2005
γ-Glutamyl Cycle and Cancer

ASCT2 transports glutamine and not glutamate, GLS1/2: glutaminase
Glutathione metabolism in cancer progression and treatment resistance 2018
Glutathione (GSH) is the most abundant antioxidant found in living organisms and has multiple functions, most of which maintain cellular redox homeostasis. GSH preserves sufficient levels of cysteine and detoxifies xenobiotics while also conferring therapeutic resistance to cancer cells. However, GSH metabolism plays both beneficial and pathogenic roles in a variety of malignancies. It is crucial to the removal and detoxification of carcinogens, and alterations in this pathway can have a profound effect on cell survival. Excess GSH promotes tumor progression, where elevated levels correlate with increased metastasis. In this review, we discuss recent studies that focus on deciphering the role of GSH in tumor initiation and progression as well as mechanisms underlying how GSH imparts treatment resistance to growing cancers. Targeting GSH synthesis/utilization therefore represents a potential means of rendering tumor cells more susceptible to different treatment options such as chemotherapy and radiotherapy.
Glutathionists in the battlefield of gamma-glutamyl cycle. 2015
- Abstract
The critical finding of our work showed that the major role of gamma-glutamyl transferase is to hydrolyze GSH and related gamma-glutamyl peptides to form free amino acids and cysteine-S-conjugates on the apical membranes of cells of various tissues and basolateral membranes of the kidney and that the resulting metabolites are transported into cells to synthesize GSH and mercapturic acids. We also showed that GSH and its S-conjugates of xenobiotics are actively secreted from cells into the circulation and/or lumenal space of the liver. The excretory transport and extracellular hydrolysis of GSH and its S-conjugates of various metabolites by gamma-glutamyl transferase and related peptidases followed by absorption of the hydrolyzed amino acids to synthesize GSH forms intra-organ and inter-organ cycles for GSH metabolism in the liver, kidney, pancreas, small intestine and other tissues that have gamma-glutamyl transferase. The series of our experiments with Helmut showed that gamma-glutamyl cycle proposed by Alton Meister does not function as the putative amino acid transporter but plays critical role in the regulation of redox metabolism of toxic free radicals and xenobiotics.