GSH Transport

Glutathione transporters. Plasma membrane GSH efflux pumps . Two GSH transporter families have been implicated in GSH efflux across the plasma membrane. The first group includes the multidrug resistant protein family (MRP) which acts as cotransporters of GSH 7 coupled to the extrusion of an OA (organic anion) and requires the use of energy. MRP proteins have also proposed to transport GSSG and GSH-conjugates. The second group includes the organic anion transporting polipeptide proteins OATP which act as GSH/OA- exchanger. This exchange is stimulated by the presence of high extracellular concentrations of OA 7 and is driven by the electrochemical gradient of GSH across the plasma membrane. Other proposed candidates for GSH efflux are hemichannels (connexins, CX), CFTR and RLIP76. Mitochondrial GSH transporters . The charged nature of GSH (negative at physiological pH) suggests that GSH cannot passively diffuse into the mitochondria, because the mitochondrial matrix has a negative potential relative to the cytoplasm. Some of the strongest candidates involved in mitochondrial import of cytosolic GSH include the dicarboxylate transporter (DIC) and the 2-oxoglutarate transporter (OGC) which act as exchangers of Pi 2 þ (inorganic phosphorous) and 2-oxoglutarate (2-OG 2 7 ) for cytosolic GSH. This transport dissipates any concentration gradient between cytoplasm and mitochondrial whose concentrations are both * 5 mM. Additionally, mitochondrial GSH transport allows this pool to be relatively independent from the cytosol, which when depleted does not affect the mitochondrial GSH pool.
The central role of glutathione in the pathophysiology of human disease, 2007
GSH Mitochondrial Transport
glutathione and mitochondrial
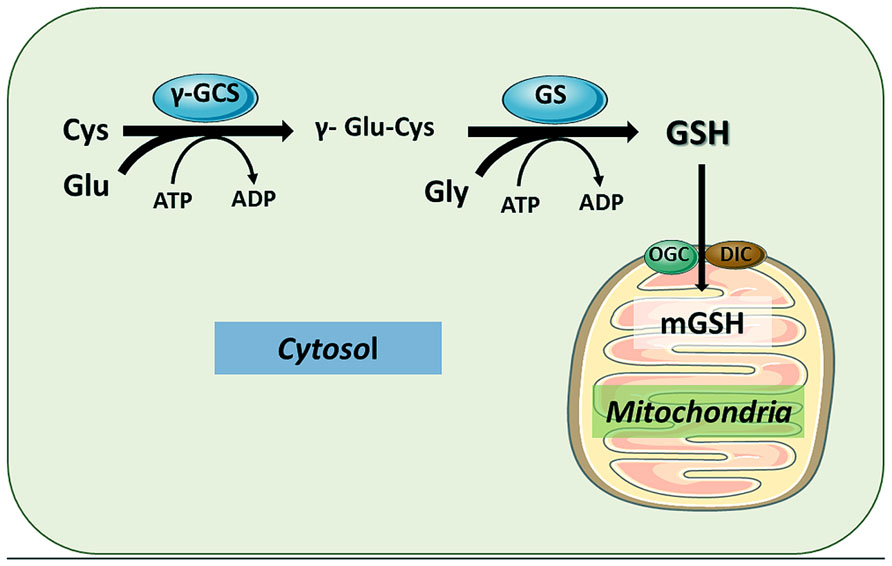
GSH can be transported to mitochondrial matrix by different carriers, particularly the 2-oxoglutarate carrier (OGC) and the dicarboxylate carrier (DIC), located in the mitochondrial inner membrane.
Glutathione and mitochondria, 2014
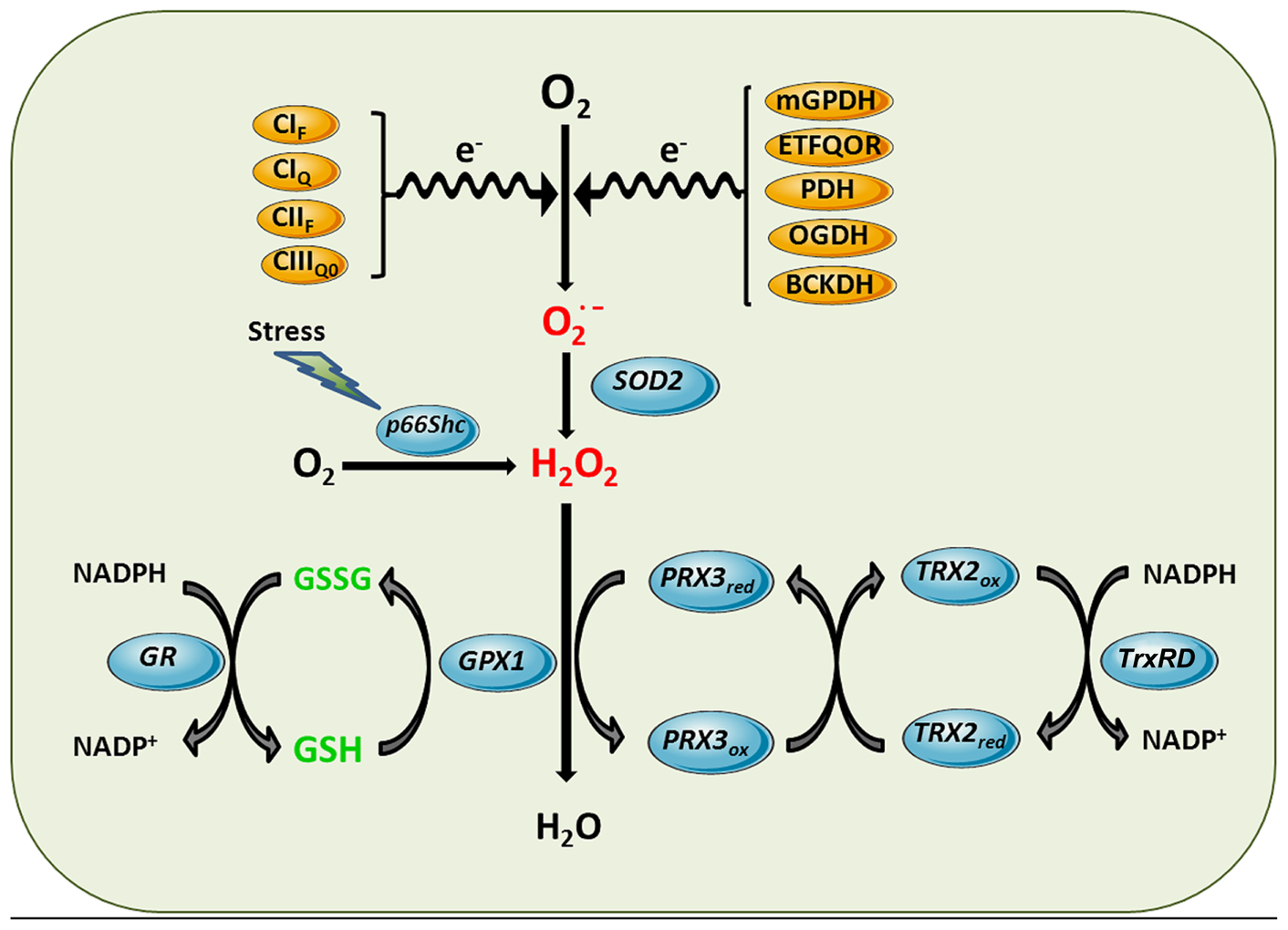
Mitochondrial ROS generation and antioxidant defense systems. Complex I flavin site (CIF), Complex I ubiquinone site (CIQ), Complex II flavin site (CIIF), and Complex IIIQo (CIIIQ0) are sites of the ETC components shown to generate superoxide anion. Other sources of superoxide can be enzymatic reactions that transfer electrons to the ETC such as mitochondrial glycerol 3-phosphate dehydrogenase (mGPDH), and the last step of β-oxidation, electron-trasferring flavoprotein ubiquinone oxidoreductase (ETFQOR) or dehydrogenases such as pyruvate dehydrogenase (PDH), 2-oxoglutarate dehydrogenase (OGDH) and branched-chain 2-oxoacid dehydrogenase (BCKDH). Superoxide generated in the mitochondrial matrix by these sites is dismutated to hydrogen peroxide by SOD2. Moreover, in response to stress p66Shc translocates to mitochondria to directly stimulate hydrogen peroxide generation by transferring electrons to cytochrome c. Hydrogen peroxide is further inactivated using the reducing equivalents of NADPH by mGSH/Gpx or Prx3/Trx2 antioxidant systems, yielding water. Mn-dependent superoxide dismutase 2 (SOD2), GSH peroxidase (GPX1), GSSG-reductase (GR), peroxiredoxin 3 (PRX3), thioredoxin-2 (TRX2), thioredoxin reductase (TrxRD).
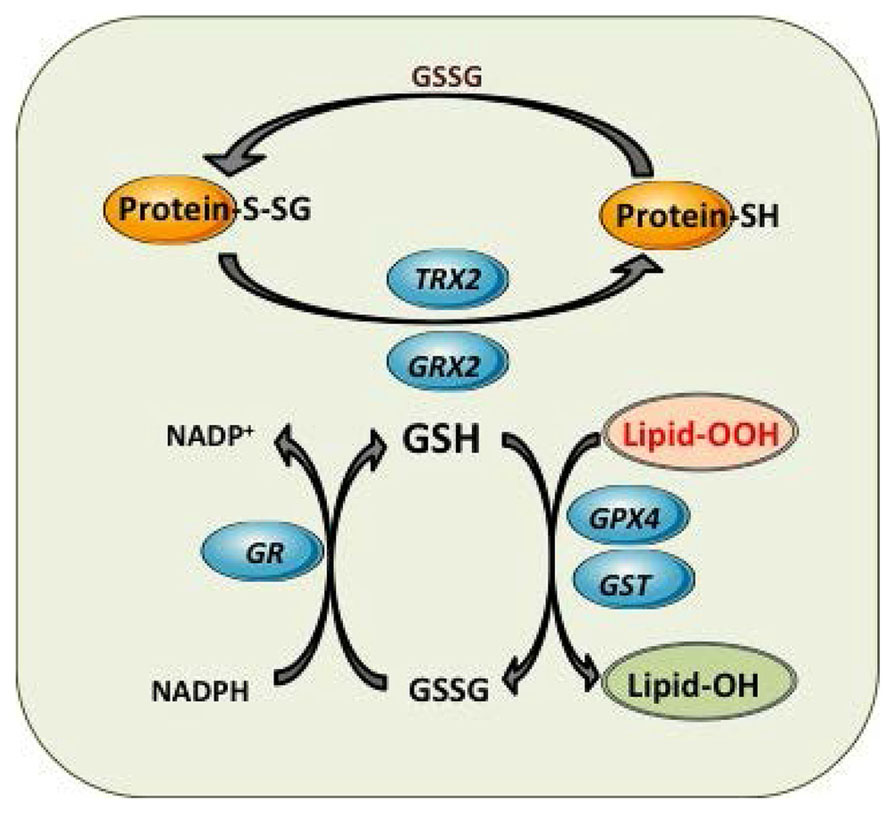
Mitochondrial GSH redox cycle and interaction with other antioxidant defenses. Detoxification of harmful lipid peroxides (Lipid-OOH) to their corresponding hydroxides (lipid-OH) by glutathione peroxidase 4 (GPX4) and glutathione-S-transferases (GST). Control of mitochondrial protein glutathionylation (Prot-S-SG, Prot -SH) by glutaredoxin (GRX2) and thioredoxin 2 (TRX2).
Increasing cytosolic GSH increases Mitochondrial GSH?
Strategies aimed to increase cytosol GSH (e.g., NAC) may not be an optimal approach for boosting mGSH and therefore for treatment of SH or AD, as it would result in mainly increasing cytosol GSH without replenishing mGSH levels
A new approach has been exploited recently in several contexts by the development of a series of cationic antioxidants targeted to mitochondria, including derivatives of the endogenous antioxidants ubiquinol (MitoQ), alpha-tocopherol (MitoVit E), and of the synthetic spin trap PBN (MitoPBN; Ross et al., 2005). These compounds have been found to block oxidative damage in isolated mitochondria and cells more effectively than untargeted antioxidant analogs due to their concentration within mitochondria. More importantly, oral administration of these compounds leads to their accumulation in the brain, heart, muscle and liver mitochondria (Ross et al., 2005). In fact, MitoQ has been used in a range of in vivo studies, in rats and mice, and in two phase II human trials demonstrating that it can be safely delivered to patients with promising results, lending further support that mitochondria-targeted antioxidants may be applicable to a wide range of human pathologies that involve mitochondrial oxidative damage (Smith and Murphy, 2010).

Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics, 2013

Mitochondrial One-Carbon Metabolism Maintains Redox Balance during Hypoxia, 2014

Glutathione--linking cell proliferation to oxidative stress. 2015
Hg and memory
Effects of mercuric chloride on spatial memory deficit-induced by beta-amyloid and evaluation of mitochondrial function markers in the hippocampus of rats, 2020
GIC
The mitochondrial dicarboxylate and 2-oxoglutarate carriers do not transport glutathione. See evidence! Twitter
twitter:Mitocarriers
14 Ways (Liposomal) Glutathione Can Boost Your Health
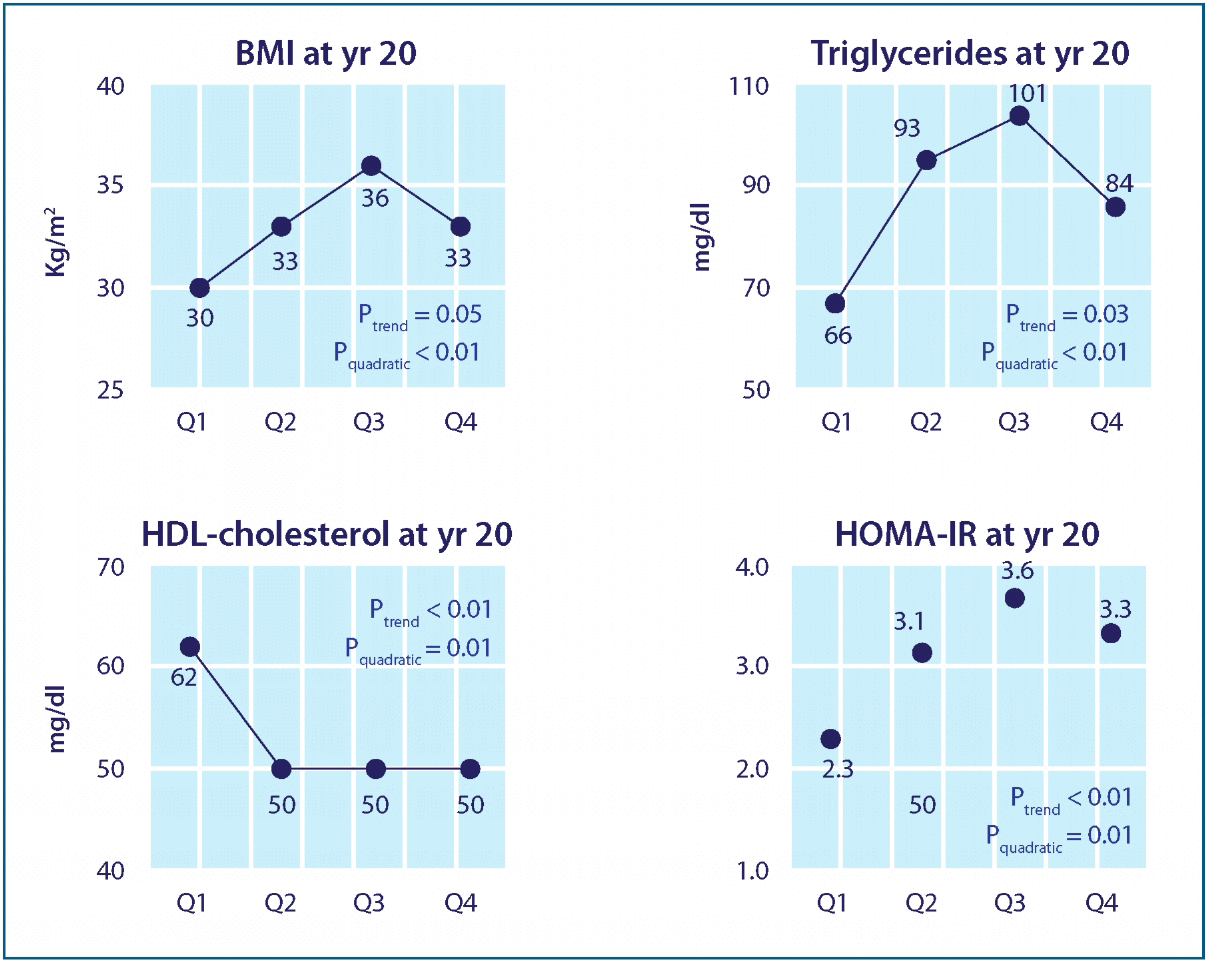
six persistent organic pollutants (POPs) measured had a dose-dependent increase in risk for diabetes
Effects of p,p’-DDE on BMI, dyslipidemia, and insulin resistance. Adjusted means of year 20 BMI, triglycerides, HDL-cholesterol, and HOMA-IR according to serum concentrations of p,p’-DDE at year 2. Adjusting variables were age, sex, race, BMI, triglycerides, and total cholesterol at year 2. Year 20 HDL-cholesterol and HOMA-IR were additionally adjusted for their baseline values at year 2 and year 7, respectively.
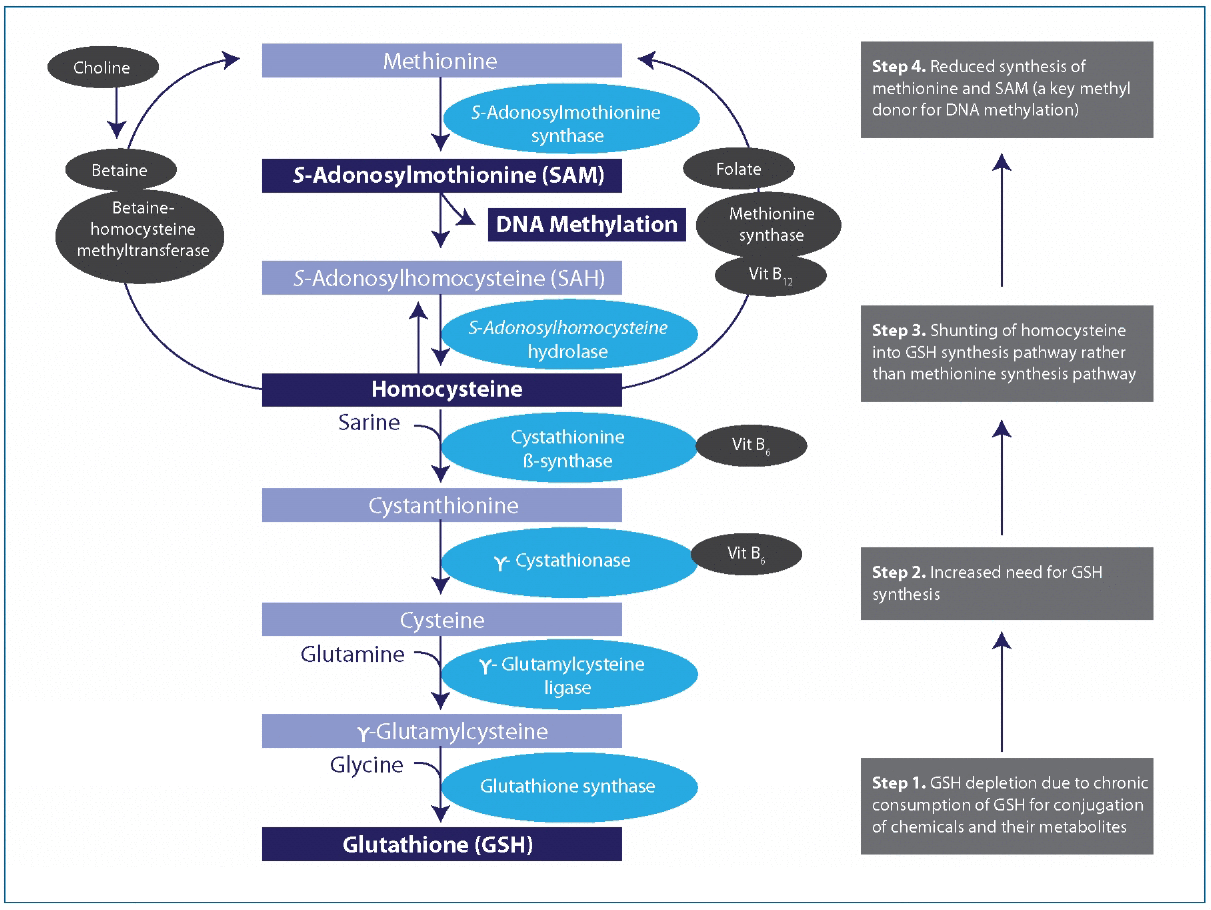
the hypothetical unifying mechanism linking DNA hypomethylation due to chemicals and nutrient deficiency or imbalance. Vit, vitamin. DNA methylation patterns can be disturbed because of the depletion of GSH when it is chronically consumed for conjugation of chemicals and their metabolites. Under usual circumstances, the metabolism of homocysteine contributes to both the methionine and GSH synthesis pathways. In the presence of chemicals such as persistent organic pollutants that deplete GSH, contribution to the methionine pathway may be diminished because of greater need to synthesize GSH (numbered boxes on the right).
Glutathione: Physiological and Clinical Relevance, 2012

The serine synthesis pathway converts 3PG to serine (Ser) through the activity of PHGDH, phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH). Ser is a substrate for mitochondrial one‐carbon metabolism, which generates NADPH while converting Ser + tetrahydrofolate (THF) into glycine (Gly) + THF + formate through the activity of serine hydroxymethyl transferase 2 (SHMT2), methylene‐THF dehydrogenase (MTHFD) 2, and MTHFD1L. Glu, glutamate; α‐KG, α‐ketoglutarate.

HIFs increase antioxidant production to maintain redox homeostasis, which is required for induction of the breast cancer stem cell phenotype under hypoxic conditions????
"The hypoxic tumor microenvironment: A driving force for breast cancer progression. 2014":
Rapid tumor regrowth resumed as soon as therapy was discontinued, whereas combination therapy with the HIF‐1 inhibitor digoxin prevented BCSC enrichment and resulted in tumor eradication (Samanta et al, 2014).
Hypoxia‐inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype, 2017