.
.
.

aging+and+Glutamate--cysteine+ligase
ROS+and+Glutamate--cysteine+ligase. molto utile
Gender difference in glutathione metabolism during aging in mice. 2003
- Oxidative damage of the macromolecules increases with age and has been suggested to contribute importantly to the aging process and the pathogenesis of many age-related diseases. However, what causes such an increase in the oxidative damage of the macromolecules and whether male and female have the same susceptibility are not clear. In this study, we demonstrated for the first time that although the concentrations of GSH were similar between young male and female mice in most tissues examined and GSH content declined with age in both genders, male mice seemed to experience more dramatic age-associated change in GSH content than did female mice in many tissues. The age-related decline in the GSH content in both male and female mice was also associated with a decrease in the amounts of glutamate cysteine ligase (GCL) mRNAs and proteins as we have reported previously in male rats, further suggesting an important role of GCL in maintaining GSH homeostasis during the aging process. The results from this study may reveal an important basis underlying the gender-associated differences in the longevity and the susceptibility to certain age-related diseases, and also further suggest that the decreased synthesis, which is mainly due to the down regulation of GCL gene expression, may be responsible for the age-associated decline in GSH content.
Anti-inflammatory activity of 8-hydroxydaidzein in LPS-stimulated BV2 microglial cells via activation of Nrf2-antioxidant and attenuation of Akt/NF-κB-inflammatory signaling pathways, as well as inhibition of COX-2 activity. 2018
Moreover, 8-OHD markedly quenched reactive oxygen species (ROS) and activated NF-E2-related factor 2 (Nrf2) so as to upregulate expression of Phase II enzymes, including heme oxygenase (HO)-1, NADH quinone dehydrogenase 1 (NQO1), and the modifier subunit of glutamate cysteine ligase (GCLM).
Glutathione metabolism in sepsis. 2007
Abstract
Sepsis is characterized by severe redox imbalance. Glutathione plays a major role in cellular defenses against oxidative and nitrosative stress. There is limited information on the response of glutathione synthesis in human sepsis. This review proposes a critical analysis of available data on potential factors affecting glutathione synthesis in sepsis. Glutathione is synthesized from its constituent amino acids--glutamate, cysteine, and glycine. Cysteine availability and the activity of the enzyme glutamate cysteine ligase are rate-limiting for glutathione synthesis. Glutathione synthetic capacity is increased in liver and other tissues during the acute phase of experimental sepsis. Potential mechanisms for glutamate cysteine ligase activation in sepsis involve a decreased ratio of reduced/oxidized glutathione as well as the effects of reactive oxygen species, nitric oxide species, proinflammatory cytokines, heat shock proteins, and physical inactivity. Glutathione synthesis can be impaired by cysteine depletion, protein-energy malnutrition, hyperglycemia, glucocorticoid at pharmacologic doses, and decreased secretion of anterior pituitary hormones (growth hormones, thyrotropin, gonadotropins), as often observed in prolonged critical illness.
Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. 2009":https://pubmed.ncbi.nlm.nih.gov/19193864/
Our results show that the NO-mediated protection toward H(2)O(2) depends on the activities of glutathione peroxidase and glutamate cysteine ligase (GCL), the rate-limiting enzyme of glutathione (GSH) de novo biosynthesis.
cyp2e1+etanolo
cyp2e1 è indotto da etanolo e poi fa piu' ROS?
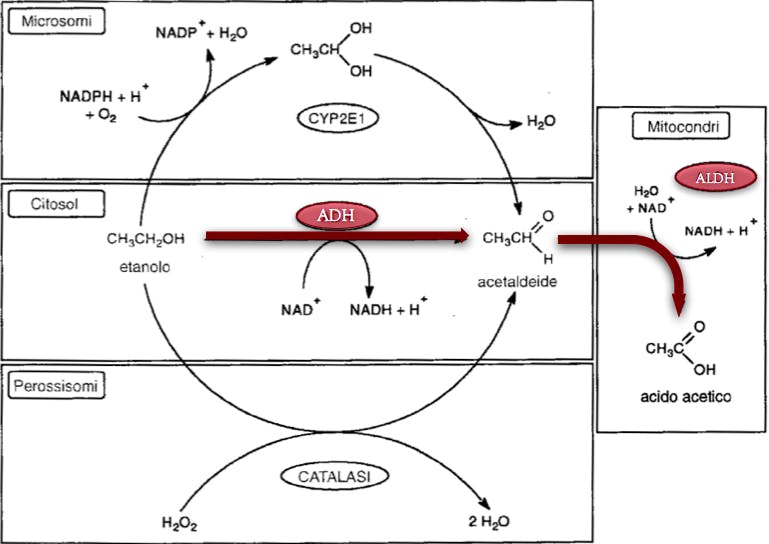
The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts, 2014
This review will focus on the effect of alcohol on CYP2E1 and its role in ROS formation, but major emphasis


CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease, 2013
Nitric Oxide-Mediated Protection of Endothelial Cells From Hydrogen Peroxide Is Mediated by Intracellular Zinc and Glutathione, 2019
CYP2E1 and ROS
expression+and+Glutamate--cysteine+ligase
hypoxia+and+Glutamate--cysteine+ligase
Hypoxia and Kinase Activity Regulate Lung Epithelial Cell Glutathione, 2010
Abstract
The authors investigated the mechanisms by which hypoxia regulates glutathione (GSH) in lung epithelial cells, and specifically whether the mitogen-activated protein kinase (MAPK) system is involved in the response to hypoxia. Hypoxia decreased cellular GSH content and appeared to decrease the effect of N-acetylcysteine on repletion of GSH after hypoxia. Hypoxia decreased 2 key enzyme activities that regulate GSH synthesis, glutamate cysteine ligase (GCL) (E.C. 6.3.2.2) and glutathione synthase (GS) (E.C. 6.3.2.3). No hypoxia-dependent change occurred in GCL or GS protein expression on Western blots. When epithelial cells were transfected with an adenoviral vector that caused over expression of human catalase protein (Ad.Cat or Ad.mCat), GCL and GS activities did not decrease in hypoxia. Inhibition of p38(MAPK) (using SB203580) or extracellular signal-regulated kinase (ERK; PD98059) prevented the hypoxia-dependent decrease in GCL and GS activity. To seek in vivo correlation, the authors assayed total glutathione in lungs and livers from MK2 (homozygous knockout) mice. MK2 mice are presumably unable to phosphorylate heat shock protein 27 (Hsp27) normally, because of absent kinase (MK2) activity. Liver GSH content (expressed per mg protein) was 20% less in MK2 mice than in nontransgenic Black 6 controls. Down-regulation of lung GSH content in hypoxia depends on peroxide tone of the cell and the p38(MAPK) system.
Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. 2008
The cardioprotective action of andrographolide was also completely abolished by buthionine sulfoximine, which acts as a specific gamma-glutamate cysteine ligase (GCL) inhibitor to deplete cellular GSH level.
Omega-3 Polyunsaturated Fatty Acids Improve the Antioxidative Defense in Rat Astrocytes via an Nrf2-dependent Mechanism, 2017
Abstract
Background: Neuronal tolerance to hypoxia and nutrient defficiency highly depends on GSH levels and antioxidant enzyme activity in astrocytes. Omega-3 polyunsaturated fatty acids (ω-3PUFA) enhance antioxidant defence in different cells. The aim of present study was to investigate if ω-3PUFA improve antioxidant status in astrocytes.
Methods: Rat primary astrocytes were incubated for 24h with DHA and EPA (30μM), then lysed, fractioned and fatty acids were determined by gas chromatography. GSH and protein thiols were assayed by enzymatic methods. Glutamate cysteine ligase (GCL), glutathione synthetase (GS), glutathione peroxidase 4 (GPx4) and Nrf2 protein expression was validated by Western blot. Intracellular ROS level using H2DCF-DA, and Nrf2 activation by ELISA were measured.
Results: Incubation of cells with DHA doubled DHA, not EPA content in the membranes, and incubation with EPA increased both fatty acids content compared to control. However, both ω-3PUFAs reduced ROS generation in dose-dependent manner in basal condition and in H2O2-treated cells, and significantly increased GSH, GCL and GPx4 levels. The thiols level was higher only in DHA-treated cells. DHA and EPA activated Nrf2 in a dose-dependent manner but p38MAPK-Nrf2 activation was found only in DHA-enriched astrocytes.
Conclusion: Both ω-3PUFA improved the antioxidant defense in astrocytes via an Nrf2-dependent mechanism, however, upstream pathways of Nrf2 activation may depend on proportion of DHA to EPA incorporated into membrane phospholipids. These results suggest that enrichment of astrocytes with ω-3PUFA may better protect neurons during harmful conditions.
Protein Aminoacids Percentage (Width 700 px)


Glutamate cysteine ligase (GCL) catalytic subunit is recent, glutathione synthetase (GS) is older and contemporary to the regulatory unit of GCL and NRf2
hypoxia+and+nrf2
hypoxia+and+nrf2+and+cell+proliferation
mTOR+and+Glutamate--cysteine+ligase
Nfr2--> mTOR--> c