DESCRIPTION
A semisynthetic antibiotic produced from Streptomyces mediterranei. It has a broad antibacterial spectrum, including activity against several forms of Mycobacterium. In susceptible organisms it inhibits DNA-dependent RNA polymerase activity by forming a stable complex with the enzyme. It thus suppresses the initiation of RNA synthesis. Rifampin is bactericidal, and acts on both intracellular and extracellular organisms.
There are various types of rifamycins from which this is derived, but the rifampicin form, with a 4-methyl-1-piperazinaminyl group, is by far the most clinically effective.
Rifampicin is an intensely red solid, and the small fraction which reaches body fluids is known for imparting a harmless red-orange color to the urine (and to a lesser extent, also sweat and tears) of users, for a few hours after a dose. Maximal concentrations in the blood are decreased by about a third when the antibiotic is taken with food
CLASSIFICATION
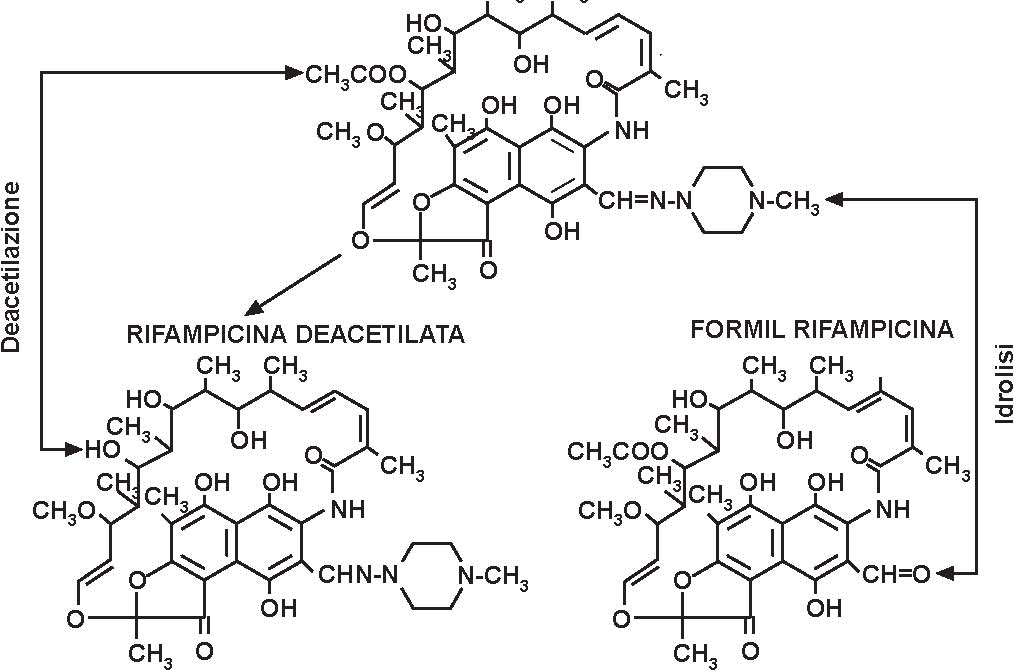
Rifampicin is a semisynthetic ansamycin derived from rifamycin B. In its structure there is a phenolic hydroxyl acid (position 1 of the naphthalene nucleus) and a piperazine basic ring. Prevails as a zwitterion at physiological pH.

INDICATION
Rifampicin was introduced in 1967, as a major addition to the cocktail-drug treatment of tuberculosis , inactive meningitis along with pyrazinamide. , isoniazid , ethambutol and streptomycin . It must be administered regularly daily for several months without break; otherwise, the risk of drug-resistant tuberculosis is greatly increased. In fact, this is the primary reason it is used in tandem with the three aforementioned drugs, particularly isoniazid. This is also the primary motivation behind directly observed therapy for tuberculosis.
Rifampicin resistance develops quickly during treatment, so monotherapy should not be used to treat these infections — it should be used in combination with other antibiotics.
Rifampicin is also used in the treatment of cholestatic pruritus
Rifampicin is typically used to treat Mycobacterium infections including Hansen's disease. It can be used to treat BCG-oma, which follows as an uncommon complication of BCG vaccination for tuberculosis.

With multidrug therapy used as the standard treatment of Hansen's disease, rifampicin is always used in combination with dapsone and clofazimine to avoid eliciting drug resistance.
Rifampicin is also effective against:
* Staphylococcus aureus
* Borrelia burgdorferi
* Anaplasma phagocytophilum
* Neisseria gonorrhoeae
* Legionella pneumophila
* Haemophilus influenzae
The Enterobacteriaceae, and Acinetobacter and Pseudomonas species are intrinsically resistant to rifampicin.
This drug is always used against active infections in combination with other antibiotics.
Rifampicin has some effectiveness against vaccinia virus
PHARMACODYNAMICS
Rifampicin inhibits bacterial DNA-dependent RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase.
Crystal structure data and biochemical data indicate that rifampicin binds to RNA polymerase at a site adjacent to the RNA polymerase active center and blocks RNA synthesis by physically preventing extension of RNA products beyond a length of 2-3 nucleotides ("steric-occlusion" mechanism).
Its lipophilic nature makes it a good candidate to treat the meningitis form of tuberculosis, which requires distribution to the central nervous system and penetration through the blood-brain barrier.
Rifampicin acts directly on messenger RNA synthesis. It inhibits only prokaryotic DNA-primed RNA polymerase, especially those that are Gram-stain-positive and Mycobacterium tuberculosis. Evidence shows that in vitro DNA treated with concentrations 5000 times higher than normal dosage remained unaffected; in vivo eukaryotic specimens' RNA and DNA polymerases suffered few problems as well. Much of this acid-fast positive bacteria's membrane is mycolic acid complexed with peptidoglycan, which allows easy movement of the drug into the cell. Rifampicin interacts with the β subunit of RNA polymerase when it is in an α2β trimer. This halts mRNA transcription, therefore preventing translation of polypeptides. It should be made clear, however, that it cannot stop the elongation of mRNA once binding to the template-strand of DNA has been initiated. The Rifampin-RNA polymerase complex is extremely stable and yet experiments have shown that this is not due to any form of covalent linkage. It is hypothesized that hydrogen bonds and π-π bond interactions between naphthoquinone and the aromatic amino acids are the major stabilizers, though this requires the oxidation of naphthohydroquinone which is found most commonly in Rifampin. It is this last hypothesis that explains the explosion of multi-drug-resistant bacteria: mutations in the rpoB gene that replace phenylalanine, tryptophan, and tyrosine with non-aromatic amino acids result in poor bonding between Rifampin and the RNA polymerase.
Resistance to rifampicin arises from mutations that alter residues of the rifampicin binding site on RNA polymerase, resulting in decreased affinity for rifampicin. Resistant mutations map to the rpoB gene, encoding RNA polymerase beta subunit.
Structural Mechanism for Rifampicin Inhibition of Bacterial RNA Polymerase, Elizabeth A. Campbell, Nataliya Korzheva, Arkady Mustaev, Katsuhiko Murakami, Satish Nair, Alex Goldfarb and Seth A. Darst, 2001
.
SIDE EFFECTS
The most serious adverse effect is related to rifampicin's hepatotoxicity, and patients receiving it often undergo baseline and frequent liver function tests to detect liver damage.
Rifampicin is an effective liver enzyme-inducer, promoting the upregulation of hepatic cytochrome P450 enzymes (such as CYP2C9 and CYP3A4), increasing the rate of metabolism of many other drugs that are cleared by the liver through these enzymes. As a consequence, rifampicin can cause a range of adverse reactions when taken concurrently with other drugs. For instance, patients undergoing long term anticoagulation therapy with warfarin have to be especially cautious and increase their dosage of warfarin accordingly. Failure to do so could lead to under-treating with anticoagulation, resulting in serious consequences of thromboembolism.
Upregulation of hepatic metabolism of hormones decreases their levels, and rifampicin can also in similar fashion reduce the efficacy of hormonal contraception , to the extent the unintended pregnancies have been reported among users of oral contraceptives taking rifampicin in even short courses (for example, as prophylaxis against exposure to bacterial meningitis).
The more common unwanted effects include fever, gastrointestinal disturbances, rashes, and immunological reactions.
Taking rifampicin can cause certain bodily fluids, such as urine and tears, to become orange-red in color, a benign side effect which can be frightening if it is not expected and prepared for. This effect may also be used to monitor effective absorption of the drug (if drug color is not seen in the urine, the patient may wish to move the drug dose farther in time from food or milk intake). The discoloration of sweat and tears is not directly noticeable, but sweat may stain light clothing orange, and tears may permanently stain soft contact lenses.
Respiratory side effects have included shortness of breath and wheezing with the use of intermittent dosage regimens. A 'flu syndrome' may appear if rifampin is taken irregularly or if daily administration is resumed after a drug free interval. Other side effects have rarely included edema of the face and extremities.
Fatal acute overdoses have been reported with doses ranging from 14 to 60 grams of rifampin. Alcohol or a history of alcohol abuse was involved in some of the fatal and nonfatal cases. The minimum acute lethal or toxic dose is not well established.
Since rifampicin may be excreted in breast milk, breast feeding should be avoided while it is being taken.
Rifampicin side effects,2013
Clinical pharmacokinetics of rifampicin,Acocella G.,1978
PHARMACOKINETICS
After oral administration on an empty stomach, the absorption of rifampicin (rifampin) is rapid and practically complete. With a single 600mg dose, peak serum concentration of the order of 10microgram/ml generally occur 2 hours after administration. The half-life of rifampicin for this dose level is of the order of 2.5 hours. The amount of rifampicin extracted by the liver during its first passage through the hepatoportal system and transferred to bile is relevance for the time course of distribution of the antibiotic in the blood compartment. With dose of the order of 300 to 450mg, the excretory capacity of the liver for the antibiotic is saturated. As a consequence, increasing the dose of antibiotic results in a more than proportional increase in serum concentrations. On repeated administration, and most likely as a consequence of self-induced (autoinduction) metabolism, the rate of disappearance of rifampicin from the blood compartment increases in the early phase of treatment, the phenomenon affecting mainly the levels following the peak, with a consequent reduction in half-life. Approximately 80% of rifampicin is transported in blood bound to plasma proteins, mainly albumin. Rifampicin is well distributed, although to a different degree, in the various tissues of the human body. Probably in the hepatocyte, rifampicin undergoes a process of desacetylation.