DEFINITION
HMGB1 (High Mobility Group Box 1, previously named HMG1 or amphoterin) is a small protein that contains 215 amino acids (25 kDa) that was discovered >30 years ago as a non-histone chromatin-associated protein involved in maintaining the architectural structure of DNA and the regulation of transcription. HMGB1 is highly conserved across species and it was named for its characteristic rapid mobility in polyacrylamide gel electrophoresis. Recently, HMGB1 as a late mediator of lethal systemic inflammation. 2001 found that HMGB1 activate an inflammatory response after its extracellular release from necrotic cells, and activated macrophages and mediate various cellular responses including chemotactic cells movement and release of proinflammatory cytokines.
THE GENE
HMGB1 is located in the long (q) arm of chromosome 13 at position 12 (13q12). This gene is composed of 8 exons that encodes 215 aa.

CHEMICAL STRUCTURE
HMGB1 has a unique structure consisting of 3 domains. The A-box and B-box domains are rich in basic amino acids and bind to DNA, while the C-terminal domain is composed of acidic amino acid clusters. It has been suggested that amino acids 6-12 bind to heparin, and that amino acids 150-183 bind to the receptor for advanced glycation end products (RAGE).


Protein Aminoacids Percentage

CELLULAR FUNCTIONS

Localization
The cellular localization of HMGB1 is tissue-specific with high levels found in the thymus, lymphoid tissues, testis, and neonatal livers. Intracellularly, HMGB1 is more concentrated in the cytoplasm of cells in liver and brain, and is concentrated in the nuclei in most other tissues
It is important to distinguish between its intracellular (as a DNA-biding protein) and extracellular (cytokine-like) function that from a certain point of view seems to be opposed.
- HMGB1 Intracellular Function
HMGB1 has been formerly known for its intracellular function – as the intranuclear non-histone DNA binding protein, which contributes to stabilization of nucleosomes, mediation of DNA bending and is regarded to have an essential position in DNA repair.
A distinguishing property of HMGB1 in the nucleus is sequence-independent DNA binding and bending, and its ability to bind preferentially to pre-bent or distorted DNA. DNA binding is a property of its two basic homologous HMG (high-mobility group)-box domains (A and B), which are linked by a short basic linker. DNA bending, towards the major groove, occurs as a result of minor-groove binding and intercalation into DNA of bulky amino acid side chains on the DNA-binding faces of the two HMG boxes. The long, homogeneously acidic, C-terminal tail (30 residues) which is linked to the boxes by a basic linker, performs a regulatory function and lowers the DNA binding affinity of the boxes. The tail is required for preferential binding to pre-bent (e.g. minicircle) DNA over linear DNA. The acidic tail also turns out to play a role in binding of HMGB1 to chromatin through interaction with core and linker histones.
HMGB1, through DNA bending, may have a chaperone role in facilitating the binding to DNA of other proteins; it interacts with a number of (unrelated) DNA-binding proteins, and this may in some cases serve to recruit HMGB1 to particular chromatin sites. HMGB1 in chromatin can contributes to chromatin structural organization.
HMGB1 also has another slightly different role in the cell nucleus, essentially acting as a chaperone that facilitates binding of various transcription factors [including Oct-1 and Oct-2, p53, Hox proteins, steroid hormone receptors and TBP (TATA-box-binding protein)] to their cognate DNA-binding site.
H1 and HMGB1: modulators of chromatin structure; 2012
Hmgb1-deficient mice are viable only for a few hours after birth, although parenterally administered glucose can prolong survival for a few days. The lack of chromosomal HMGB1 protein does not disrupt cell growth, but may affect the transcriptional regulation of certain genes, such as those activated by the glucocorticoid receptor.
- HMGB1 Extracellular Function
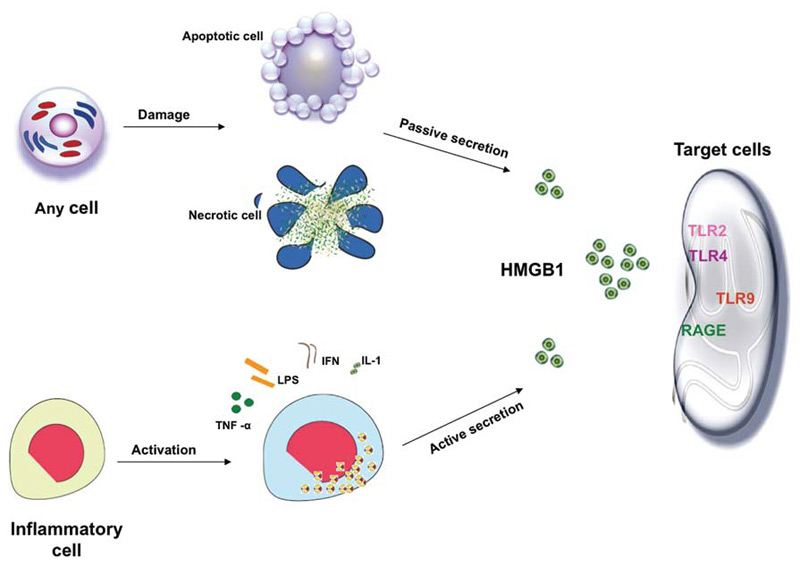
Under inflammatory or injurious conditions, HMGB1 can be released actively by innate immune cells (macrophages, monocytes), and passively by necrotic cells.
Receptor for advanced glycation end-products (RAGE) was the first receptor identified for extracellular HMGB1 but other receptors, such as Toll-like receptor 2 (TLR2) and TLR4, have also been shown to contribute to HMGB1-mediated signaling. HMGB1 induces NF-kB activation and release of pro-inflammatory cytokines and chemokines such as TNF, IL-1, IL-6 and IL-8.
Active Release
In response to stimulation with exogenous bacterial endotoxin (e.g. lipopolysaccharide, LPS) or endogenous proinflammatory cytokines such as tumour necrosis factor (TNF), interleukin (IL)-1b, interferon (IFN)-gamma, cultures of macrophages, monocytes and pituicytes actively release HMGB1 in a time and dose-dependent manner. HMGB1 release by activated macrophages is not due to cell death, but dependent on active translocation of HMGB1 from the nucleus to the cytoplasm. Following stimulation with IFN-gamma, cellular HMGB1 is observed predominantly in the cytoplasm as aggregated granules, raising the possibility that nuclear HMGB1 translocates to the cytoplasm before being released into the extracellular milieu. Similarly, in response to TNF stimulation, human synovial fluid macrophages translocate HMGB1 from the nucleus to the cytosol. As TNF, IL-1b and IFN-gamma mediated inflammatory effects are often highly synergistic, it is plausible that such a synergistic interaction between TNF, IL-1b and IFN-gamma may be involved in the regulation of HMGB1 release during systemic inflammation. HMGB1 lacks a classical signal sequence in the N-terminus,and the mechanisms underlying the regulation of HMGB1 release remain largely unknown. However, it may be released through an atypical endolysosomal-like secretory pathway in human monocytes.
Passive Release
In addition to active release from activated innate immune cells, HMGB1 can also be passively released by necrotic or damaged cells. HMGB1 released by necrotic cells is capable of inducing an inflammatory response, thereby transmitting the ‘injury’ signal to neighbouring immune cells. Therefore, HMGB1 might be a critical molecule that allows innate immune cells both to respond to injury, and to further induce inflammation. However, HMGB1 is not released by apoptotic cells even after subsequent secondary necrosis and partial autolysis. Thus, cells undergoing apoptosis fail to activate inflammatory responses, whereas cells undergoing necrosis release HMGB1 and activate inflammatory responses during tissue injury and trauma.
Extracellular role of HMGB1 in inflammation and sepsis. 2004
HMGB1 in Inflammatory Diseases
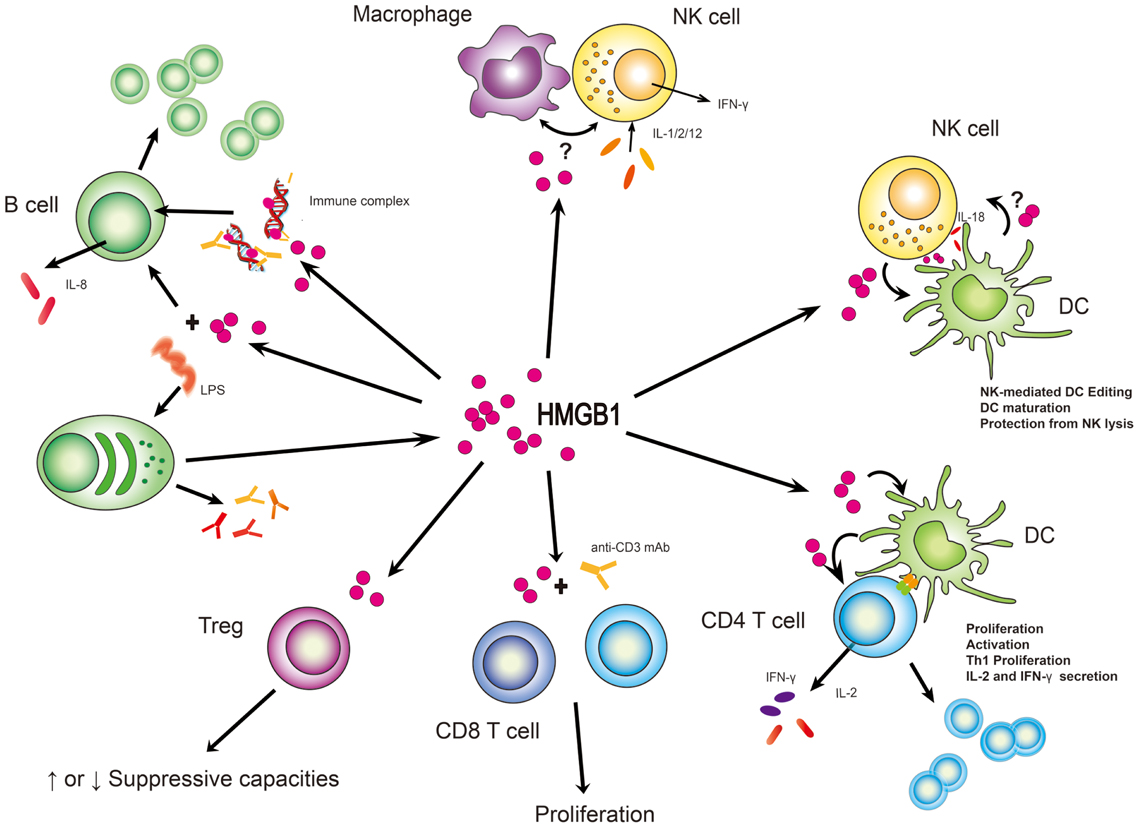
The recent discovery of the extracellular role of HMGB1 as a proinflammatory cytokine has opened up a new field of research to study the role of HMGB1 in inflammatory diseases, including severe sepsis and arthritis. HMGB1 is unique in its delayed release kinetics, suggesting that it may be an important therapeutic target in lethal systemic inflammatory diseases in which excessive amounts of HMGB1 are released. HMGB1 is found in the synovium of arthritic joints of humans, and specific inhibition of HMGB1 activity attenuates the development of murine experimental arthritis. These and other observations indicate that HMGB1 is an important mediator of local and systemic inflammatory diseases. The kinetics of HMGB1 release is delayed compared with most other cytokines. This delayed HMGB1 response is different from TNF and distinguishes HMGB1 from other early-acting, proinflammatory cytokines.
Sepsis
Serum HMGB1 levels were significantly increased in 25 sepsis patients compared with healthy volunteers, and levels were found higher in patients succumbed to the disease than in survivors. Studies are in progress to elucidate whether elevated HMGB1 levels in sepsis patients are predictive of prognosis.
In murine models of endotoxemia and (Cecal Ligation and Punture) CLP-sepsis, HMGB1 is first detected in the circulation eight hours after the disease onset, and subsequently, increased to plateau levels from 16 to 32 hours. This late appearance of circulating HMGB1 parallels with the onset of animal lethality from endotoxemia or sepsis and distinguishes itself from TNF and other early proinflammatory cytokines.
The pathogenic role of HMGB1 in endotoxemia was inferred from studies using HMGB1-neutralizing antibodies, which conferred a dose-dependent protection against endotoxin-induced tissue injury and lethality. In a more clinically relevant animal model of sepsis (induced by CLP), delayed administration of HMGB1-specific neutralizing antibodies beginning 24 h after CLP, dose-dependently rescued rodents from lethal sepsis.
The discovery of HMGB1 as a late mediator of experimental sepsis has stimulated a great interest in developing neutralizing antibodies for clinical management of human sepsis. In the animal model of sepsis, monoclonal antibodies effectively rescued mice from the lethal sequelae even when given 24 h post the onset of sepsis. It is not yet known whether HMGB1 can ever be a clinically feasible therapeutic target for human sepsis until HMGB1-neutralizing antibodies are tested in clinical trials. Targeting HMGB1 in the treatment of sepsis. 2013
HMGB1 antibodies inhibit endotoxin lethality in mice and also inhibit lung inflammation following airway LPS exposure.RAGE-deficient mice are also protected from endotoxemia, suggesting that HMGB1-induced lethality is mediated through RAGE signaling.
Systemic Lupus Erythematosus
Increased concentrations of HMGB1 were found in the serum and plasma from a substantial number of patients with SLE. A more recent study shows that HMGB1-nucleosome complexes activate antigen presenting cells and, thereby, may crucially contribute to the pathogenesis of SLE via breaking the immunological tolerance against nucleosomes/dsDNA. Besides the pathological roles of HMGB1 in SLE and other autoimmune diseases, HMGB1 itself can be the target of an autoimmune response: anti-HMGB1 antibodies were found in patients with several autoimmune diseases including systemic sclerosis, ulcerative colitis, juvenile idiopathic arthritis, and SLE.
HMGB1 and RAGE activate plasmacytoid dendritic cells and B cells in response to DNA and contribute to autoimmune pathogenesis. In a recent work the authors reported that HMGB1 may act as a proinflammatory mediator in antibody-induced kidney damage in SLE.
HMGB1 may play an important role in the pathogenesis of SLE, but its exact mechanism remains to be determined. High Mobility Group Box 1: a potential therapeutic target for systemic lupus erythematosus. 2009
Arthritis
In chronic inflammatory diseases such as arthritis, elevated HMGB1 levels have been reported in synovial fluid in experimental arthritis animal models as well as in human patients with rheumatoid arthritis. In immunohistochemistry studies, tissues sections from synovitis patients showed the presence of cytoplasmic and extracellular HMGB1. This pattern of staining is significantly different from normal synovial tissue, which shows HMGB1 strictly localized in the nucleus. Administration of HMGB1 into mice joints also induced arthritis changes and stimulated the synovial macrophages to release proinflammatory cytokines including TNF, IL-1, and IL-6.
Considered together, these data indicate that HMGB1 plays a pathogenic role in arthritis. These observations raise the possibility that HMGB1 may serve as a marker, as well as a mediator, of critical illness in the absence or presence of infection.
Trauma and Sterile Inflammation
HMGB1 levels are elevated in patients with mechanical trauma, strokes, acute myocardial infarction, acute respiratory distress, and liver transplantation.
HMGB1 and Cancer
The involvement of HMGB1 in cancer is complex, and intracellular/nuclear and extracellular forms of HMGB1 have been implicated in tumor formation, progression, and metastasis and in the response to chemotherapeutics. Elevated expression of HMGB1 occurs in several solid tumors, including melanoma, colon cancer, prostate cancer, pancreatic cancer, and breast cancer. In gastrointestinal stromal tumors,the overexpression of HMGB1 is closely associated with gain-of-function mutations in c-kit. Importantly, HMGB1 in cancer is associated with invasion and metastasis. HMGB1 may be directly involved in tumor cell metastasis through its ability to promote cell migration, to modulate the adhesive properties of cells, and to modify com- ponents of the extracellular matrix. In certain cases, however, the effect may be indirect, and, by enhancing the activity of NF-κB p65, HMGB1 leads to the induction of melanoma inhibitory activity (MIA), an 11-kDa secreted protein, which binds to fibronectin as well as to certain integrins and enhances migration and invasion of tumor cells. Thus, HMGB1 does not necessarily have to be secreted by tumor cells to enhance their invasive and metastatic potential.
As a cytokine,HMGB1 not only stimulates inflammatory cells,but also can activate vascular endothelial cells.HMGB1 can stimulate endothelial cell proliferation and sprouting in vitro and neovascularization in vivo. HMGB1 and RAGE in inflammation and cancer. 2010
HMGB1 and Virus

HMGB1 and skin
HMGB1 the chemical that makes bone marrow to repair skin
