INTRODUCTION
The Opioids are a class of controlled pain-management drugs that contain natural or synthetic chemicals based on morphine, the active component of opium. These narcotics effectively mimic the pain-relieving chemicals that the body produces naturally.

CLASSIFICATION
There are a number of broad classes of opioids:
- Natural opiates (alkaloids contained in the resin of the opium poppy, primarily morphine, codeine, and thebaine),
- Esters of morphine
- Semi-synthetic opioids (created from either the natural opiates or morphine esters, such as hydromorphone, hydrocodone, oxycodone, oxymorphone, ethylmorphine and buprenorphine)
- Fully synthetic opioids (such as fentanyl, pethidine, levorphanol, methadone, tramadol and dextropropoxyphene)
- Endogenous opioid peptides (produced naturally in the body, such as endorphins, enkephalins, dynorphins, and endomorphins).
1 - (Opioids classification)
MODE OF ACTION
All opioid analgesics mimic endogenous (meaning produced by the human body) endorphins by stimulating opioid receptors μ, κ, δ (mu, kappa, and delta) in the central and peripheral nervous systems which results in relief of pain. The pharmacodynamic response to an opioid depends upon the receptor to which it binds, its affinity for that receptor, and whether the opioid is an agonist or an antagonist. Opioids are particularly useful in pain management as they:
• Can be given by a variety of routes including oral, transmucosal, rectal, intravenous, subcutaneous, intramuscular, transdermal, epidural and intrathecal;
• Are easily titrated to the correct dose
• Are highly effective
• Have a favourable risk benefit ratio

1 - (Mechanism of action)
IT’S NOT ALL A BED OF ROSES…
These drugs are widely used to alleviate cancer pain. In addition, the perioperative management of pain in cancer surgery patients most often includes opioids. However, there are reports that these drugs may alter cancer recurrence or metastasis. Several mechanisms have been proposed:
- OPIOIDS MODULATE ANGIOGENESIS
Endothelial cells express opioid receptors and respond to opiates by increasing intracellular calcium concentration and nitric oxide production. In addition to vascular permeability, nitric oxide regulates endothelial cell proliferation, migration, and protease release, all of which are important for angiogenesis. Clinically relevant concentrations of morphine result in the activation of mitogen-activated protein kinase/ extracellular signal-regulated kinase MAPK/ERK and pro- survival Akt in cultured endothelial cells. Morphine was also shown to induce endothelial cell proliferation and migration via the transactivation of growth factor receptors that play a key role in migration and survival of these cells, including the vascular endothelial growth factor (VEGF) receptor 2 or the platelet-derived growth factor (PDGF)-beta receptor. In a non-endothelial cell system, morphine was further shown to transactivate the receptor (FGFR1) of another growth factor playing a key role in angiogenesis, namely fibroblast growth factor (FGF) .
One mechanism through which the VEGF pathway increases endothelial cell migration involves adhesion molecules, especially intercellular adhesion molecule 1 or ICAM-1: VEGF stimulation of endothelial cells increases ICAM-1 expression which is reported to not only increase endothelial cell migration but also to mediate the recruitment of endothelial progenitor cells, which participate in angiogenesis.
Another actions key factor that promotes angiogenesis is the activation of cyclooxygenase-2 (COX-2), increasing production of prostaglandin E2, which promotes angiogenesis and tumor progression.

1 - (Morphine and tumor growth and metastasis.2011)
- OPIOIDS ARE IMMUNOSUPPRESSIVE
The vast majority of reports indicate that morphine and other exogenous opioids are immunosuppressive. The immune system plays a key role in the control of tumor formation and metastasis. Tumor cells express non-self- antigens and are subjected to killing by activated T and NK cells and to the cytotoxic action of cytokines such as interferon gamma.
Moreover, after cancer cells have escaped immunosurveillance and a tumor develops, innate and adaptive immune cells are also present in the tumor microenvironment where they can promote or fight tumor development.
Oppioid receptors have been identified in cells of the immune system such as polymorphonuclear leukocytes, macrophages, T lymphocytes, splenocytes, macrophage - like, and T cell-like cell lines: acute and chronic administration of exogenous opioids including the one most commonly used—morphine —inhibits components of the cellular and humoral immune function such as antibody production, NK cell activity, cytokine expression, blood lymphocyte proliferative responses to mitogen, and phagocytic activity.
The effects of opioids on Polimorphonuclear cells seem to be related to the inhibition of Ca2+ influx mediated Kop receptors. but it has been observed that kappa agonists may interfere with the Ca2+ regulatory system of PMNs via opioid receptor non-mediated pathway. The effect was suspected to be caused by selective inhibition of Ca2+ release from intracellular Ca2+ store sites by affecting post-receptor mechanisms.
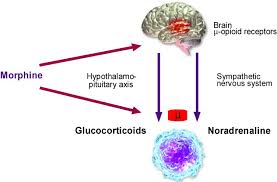
Opioids are now known to modulate the immune response by a combination of central and peripheral mechanisms. Signals of central origin may be relayed through (1) the hypothalamic–pituitary–adrenal (HPA) axis, resulting in the production of glucocorticoids which are immunosuppressive, and (2) the sympathetic nervous system eliciting the release of biologic amines into lymphoid organs, which also reduce immunocompetence.
Ample evidence exists for the involvement of catecholaminergic pathways in alteration of opioid-mediated changes in immune function. Biochemical and histochemical studies demonstrate a rich noradrenergic input to the bone marrow, thymus, spleen, lymph nodes and gut associated lymphoid tissue In addition, noradrenergic terminals have been observed in direct apposition to lymphocytes.
Catecholamines released upon sympathetic nervous system activation stimulate target cells by interacting with cell surface adrenergic receptors (adrenoreceptors). There are two major classes of adrenoreceptors, α and β. α1-Adrenoreceptors stimulate phosphatidylinositol turnover and induce a rise in intracellular calcium; α2-adrenoreceptors are linked to the Gi subunit of the adenylate cyclase complex inhibiting cyclic AMP (cAMP). On the other hand, β-adrenoreceptors are coupled intracellularly to the GTP-binding protein, Gs, of the adenylate cyclase complex, giving rise to increased cAMP upon stimulation.
Adrenoreceptors have been associated with immunoregulation, and β-agonists have been shown to inhibit anti-CD3-induced T-cell proliferation and IL-2 production, NK-cell cytotoxicity, LPS-induced TNF-α production, expression of nitric oxide synthase, and macrophage phagocytosis and respiratory burst.
β-agonists are well known to increase the intracellular levels of cAMP suppressing lymphocyte and macrophage functions. Release of endogenous catecholamines inhibits T helper cell responses and can be blocked by combined α- and β-adrenoreceptor antagonists. However, little is known about the modulation of other second messengers or gene expression following adrenoreceptor stimulation.
Regarding instead corticosteroids,they exert their effects by binding to a cytoplasmic receptor which has several functional domains including a ligand-binding domain, a DNA-binding domain and two domains that are involved in transactivation of genes once binding to DNA, increasing the
synthesis of anti-inflammatory proteins, such as annexin-1, IL-10, MAPK phosphatase-1 (MKP-1) and the inhibitor of NF-kB, I-kB.
1 - (Surgery for cancer: does anesthesia matter?.2010)
2 - (Current knowledge of Buprenorphine and its unique pharmacological profile.2010)
3 - (Differential effects of buprenorphine and morphine on immune and neuroendocrine functions.2000)
4 - (Regulatory T cells: a possible promising approach to cancer recurrence induced by morphine.2013)
NOT ALL DRUGS ARE THE SAME…
The immunomodulatory effects of morphine have been characterized in animal and human studies.
Morphine decreases the effectiveness of several functions of both natural and acquired immunity, interfering with important intracellular pathways involved in immune regulation. Morphine significantly suppressed splenic NK cell cytotoxic activity (14-50% reduction), splenic and thymic T cell proliferation to concanavalin A, antiTCR (T cell receptor) (85% reduction) and IL-2 (36-48% reduction), and macrophage functions including nitric oxide (36-41% reduction), TNF-alpha production (26%), and phagocytosis of Candida albicans (39%). On the other side morphine increase glucocorticoid and catecholamine levels in plasma and spleen dialysates, respectively. These results indicated that morphine activate either the hypothalamic-pituitary-adrenal (HPA) axis with glucocorticoid release, or the sympathetic nerve (SNS) activity with bioamine production, and was associated with immunosuppression. Morphine could also induce cancer recurrence by disturbing the behavior of the regulatory T cells.
Mainly from animal studies, however, it has emerged that not all opioids induce the same immunosuppressive effects and evaluating each opioid's profile is important for appropriate analgesic selection.
There are, from a molecular structural aspect, some opioids which have a carbonyl substitution at C6, a single bond between C7-8 and, preferably, an hydroxyl group at C14, which can be considered the most clinically acceptable: they are buprenorphine, hydrocodone, oxycodone, oxymorphone and tramadol, which not only have no immunosuppressant properties but may have additional pharmacological and clinical advantages over the immunosuppressant group of opioid analgesics.
For example Buprenorphine is an analgesic recently approved for the treatment of drug dependency. Several studies have shown that a standard dose of buprenorphine did not alter splenic NK cell, T cell, and macrophage functions. In addition, buprenorphine was associated with significant reductions in adrenocorticotropic hormone (ACTH) and corticosterone (CSO) plasma levels, without altering norepinephrine (NE) and serotonin splenic dialysate levels. These results indicated that buprenorphine did not activate either the hypothalamic-pituitary-adrenal (HPA) axis with glucocorticoid release, or the sympathetic nerve (SNS) activity with bioamine production, and was not associated with immunosuppression. The lack of effects of buprenorphine on neuroendocrine systems may be related to its partial agonist properties and to the absence of effects on immune system function.
1 - (Differential effects of buprenorphine and morphine on immune and neuroendocrine functions.2000)
CONCLUSION
Several studies provided evidence that morphine can affect tumor growth by acting with different mechanisms, including tumor cells or endothelial cells or growth factors secreted by meditation of CNS. Some reports suggested that morphine may promote the tumor growth by inhibiting apoptosis and by promoting angiogenesis and migration of tumor cells.
Anyway the role opioids play in the development of cancer metastasis and recurrence is far from clear and appears to differ depending on the cancer cell type in question and on the type of drug used.
1 - (Opioids and cancer recurrence.2014)
2 - (Does regional anaesthesia and analgesia or opioid analgesia influence recurrence after primary cancer surgery?.2013)
3 - (Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis?.2012