Humans have adrenals that secrete large amounts of the inactive precursor steroids dehydroepiandrosterone ( DHEA ) and especially DHEA-sulfate ( DHEA-S ), which are converted into potent androgens and/or estrogens in peripheral tissues (Intracrinology. 1991, DHEA and peripheral androgen and estrogen formation: intracrinology.l. 1995, 1997d, 2000b, 2001, Analysis and characteristics of multiple types of human 17beta-hydroxysteroid dehydrogenase):

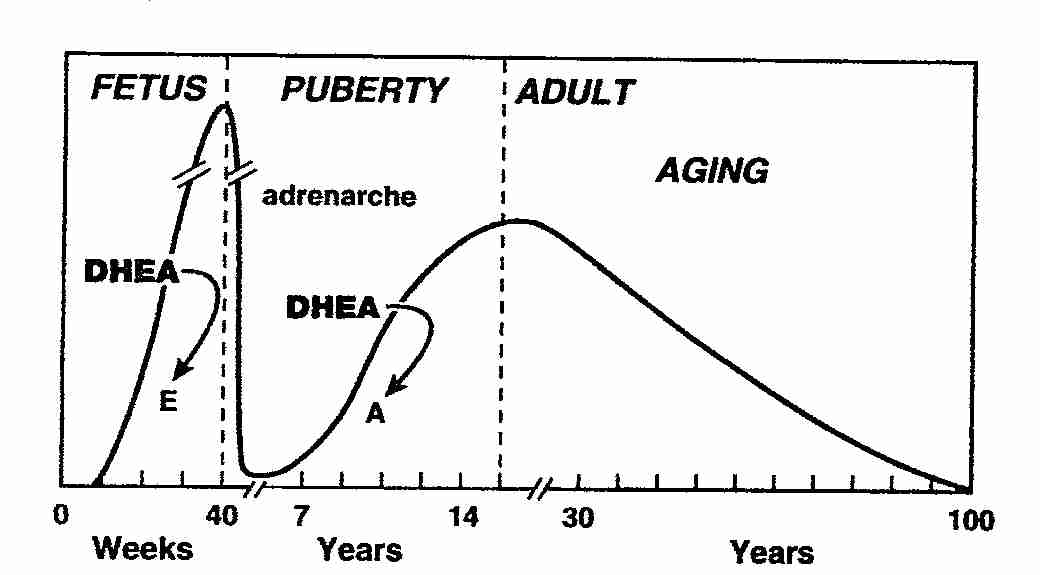
Adrenal secretion of DHEA and DHEA-S increases during adrenarche in children at the age of 6–8 years. Maximal values of circulating DHEA-S are reached between the ages of 20 and 30 years. Thereafter, serum DHEA and DHEA-S levels decrease markedly:

a deeper insight
The marked reduction in the formation of DHEA-S by the adrenals during aging (Radioimmunoassay [...], Adrenocortical Function in Old Age: Response to Acute Adrenocorticotropin Stimulation 1982, Age Changes and Sex Differences in Serum DHEA-S Concentrations throughout Adulthood 1984, Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men 1994, Marked Decline in Serum Concentrations of Adrenal C19 Sex Steroid Precursors and Conjugated Androgen Metabolites During Aging 1997c) results in a dramatic fall in the formation of androgens and estrogens in peripheral target tissues, a situation that has been proposed to be associated with age-related diseases such as insulin resistance (Therapeutic effects of DHEA in diabetic mice 1982, DIVERGENT CORRELATIONS OF CIRCULATING DHEA-S AND TESTOSTERONE WITH INSULIN LEVELS AND INSULIN RECEPTOR BINDING 1988) and obesity (DHEA Reduces Serum Low Density Lipoprotein Levels and Body Fat but Does not Alter Insulin Sensitivity in Normal Men 1988, Reduced testosterone and adrenal C19 steroid levels in obese men).
Why man, in addition to possessing very sophisticated endocrine and paracrine systems, has largely invested in sex steroid formation in peripheral tissues?
In fact, while the ovaries and testes are the exclusive sources of androgens and estrogens in lower mammals, the situation is very different in man and higher primates, where active sex steroids are in large part or wholly synthethized locally in peripheral tissues, thus providing target tissues with the appropriate controls which adjust the formation and metabolism of sex steroids to local requirements. (Intracrinology 1991)
DHEA and DHEA-S metabolism in peripheral tissues
Transformation of the adrenal precursor steroids DHEA-S and DHEA into androgens and/or estrogens in peripheral target tissues depends upon the level of expression of the various steroidogenic and metabolizing enzymes in each cell of these tissues:

DHEA metabolism in men
Although orchiectomy, estrogens or gonadotropin-releasing hormone (GnRH) agonists or antagonists (through blockade of secretion of bioactive LH) cause a 90–95% reduction in the concentration of circulating testosterone (Fig. below), a much smaller effect is seen on the only parameter that directly reflects intra-tissular androgenic action, i.e. the intra-prostatic concentration of the potent androgen DHT. In fact, intra-prostatic DHT levels are reduced by only 50–70% following medical or surgical castration (Fig. below):

DHEA metabolism in women
In women, the role of the adrenal precursors DHEA-S, DHEA and 4-dione in the peripheral formation of estrogens is even more important than the situation in men for androgens. In fact, in men, androgen secretion by the testes continues at a high level through life while, in women, estrogen secretion by the ovaries completely ceases at menopause, thus leaving the adrenals as the only source of sex steroids. In fact, the best estimate is that the intracrine formation of estrogens in peripheral tissues in women accounts for 75% of all estrogens before menopause, and close to 100% after menopause (Control of secretion and the function of C19-delta 5-steroids of the human adrenal gland 1985, Endocrine and Intracrine Sources of Androgens in Women: Inhibition of Breast Cancer and Other Roles of Androgens and Their Precursor DHEA 2003a).
Much attention has been given to the benefits of DHEA administered to postmenopausal women, especially on the bone, skin, vaginum and well-being after oral (Effects of replacement dose of DHEA in men and women of advancing age 1994, DHEA, DHEA-S, and aging: Contribution of the DHEAge Study to a sociobiomedical issue 2000) and percutaneous (Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women 1996, Effect of 12-Month DHEA Replacement Therapy on Bone, Vagina, and Endometrium in Postmenopausal Women 1997b) administration.
 (Fig. Comparison of the effects of standard ERT (estrogen) and DHEA on parameters of menopause.)
(Fig. Comparison of the effects of standard ERT (estrogen) and DHEA on parameters of menopause.)
Other DHEA targets
The skin is also an important target of intracrine sex steroid action. In fact, it is well recognized that the skin synthesizes androgens from inactive steroid precursors and that acne, seborrhea, hirsutism and androgenic alopecia are associated with excess androgens (In Vivo Studies on Testosterone Metabolism by Skin of Normal Males and Patients 1969, THE METABOLISM OF TESTOSTERONE BY HUMAN MALE SCALP SKIN 1973, Androgens: Pharmacodynamics and antagonists. Biochemical and biological studies with 4-aza-steroidal 5 alpha-reductase inhibitors 1983, Characterization, expression, and immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase in human skin 1992b, Comparison of flutamide and spironolactone in the treatment of hirsutism: a randomized controlled trial 1994). In fact, increased local biosynthesis of the potent androgen DHT from the weaker androgen testosterone by 5 -reductase has been suggested to be one of the mechanisms involved (Male Pseudohermaphroditism: A Comparative Study of One Patient with 5{alpha}-Reductase Deficiency and Three Patients with the Complete Form of Testicular Feminization 1979). Although a series of studies have addressed the role of sex steroids in the control of hair growth and sebaceous gland physiology, the importance of skin as a site of regulated steroid biosynthesis and metabolism has received little attention.
It should also be noted that the importance of the intracrine formation of androgens and estrogens extends to osteoporosis, vaginal atrophy and endometriosis (Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance 2000). In this regard, it has recently been demonstrated that aromatase is expressed aberrantly in endometriosis, while this activity is not detectable in the normal endometrium.