DEFINITION
A short yet comprehensive description
External links
| Database | Link |
| "XYZZ": | "test": |
| "ZXVX": | "URL": |
ANALYTICAL METHOD
ANALYTICAL TRICKS AND TIPS
THE BIOLOGICAL CONTEXT

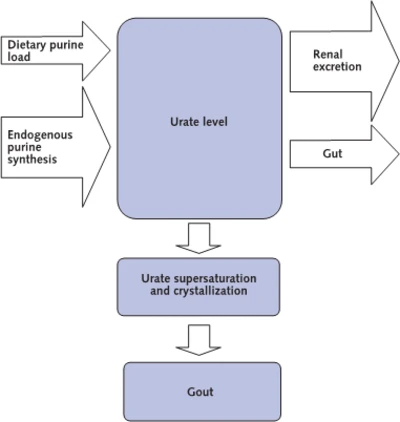
Gout Development
THE NORMAL RANGE
DIAGNOSTIC USE
Issues
Specificity, sensitivity etc.
Diagnostic Algorithms
PROs and CONTROs
Open Questions
Association between vitamin D insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. 2013
Vitamin D insufficiency was significantly associated with elevated uric acid among postmenopausal Chinese Han women.
Stabilita associazione tra livelli di acido urico e diabete
Livelli sierici elevati di acido urico risulterebbero associati allo sviluppo di diabete di tipo 2. Sono i risultati di un'ampia metanalisi pubblicata da un gruppo di ricercatori giapponesi su Diabetes Care. L'indagine ha preso in considerazione ben 11 studi clinici, presenti nei database Medline (dal 1966 al 2009) ed Embase (dal 1980 al 2009), riguardanti oltre 42mila pazienti diabetici e con follow-up compresi tra 2,0 e 13,5 anni. In sintesi, gli autori hanno calcolato un rischio relativo di sviluppare diabete pari a 1,17 per ogni incremento di 1 mg/dL di acido urico nel siero. "Trattandosi di studi osservazionali non è stato possibile stabilire una relazione causa-effetto tra livelli di acido urico e diabete" ha commentato Hirohito Sone del Department of Internal Medicine, University of Tsukuba di Ibaraki. "Per stabilire se i livelli di questa sostanza possano rappresentare un fattore predittivo dello sviluppo di diabete sarà necessario valutare non solo la suddetta relazione causa-effetto ma anche i valori di cut-off di acido urico". (L.A.).
Diabetes Care 2009, 32, 1737-1742
Serum uric acid is associated with bone health in older men: A cross-sectional population-based study. 2011 J Bone Miner Res. 2011 May;26(5):955-64. doi: 10.1002/jbmr.286.
Serum uric acid (UA) is a strong endogenous antioxidant. Since oxidative stress has been linked to osteoporosis, we examined the association between serum UA levels and bone mineral density (BMD), prevalent vertebral and nonvertebral fractures, and laboratory measures such as calcitropic hormones and bone turnover marker levels. This cross-sectional analysis consisted of 1705 community-dwelling men aged 70 years or over who participated in the baseline part of the Concord Health and Ageing in Men Project (CHAMP), a population-based study of older men in Sydney, Australia. BMD at all sites was significantly higher among men with serum UA levels above the group median than among men with UA levels below the median. In multiple regression analyses adjusted for potential confounders, serum UA remained associated with BMD at all sites (β = 0.12 to 0.14, p < .001), serum calcium (β = 0.11, p = .001), parathyroid hormone (β = 0.09, p = .002), 25-hydroxyvitamin D (β = 0.09, p = .005), and was negatively associated with urinary excretion amino-terminal cross-linked telopeptide of type 1 collagen (β = -0.09, p = .006). Overall, serum UA accounted for 1.0% to 1.44% of the variances in BMD (R(2) = 0.10 to 0.22). In multiple logistic regression analyses, above-median serum UA levels were associated with a lower prevalence of osteoporosis at the femoral neck [odds ratio (OR) = 0.42, 95% confidence interval (CI) 0.22-0.81, p = .010) and lumbar spine (OR = 0.44, 95% CI 0.23-0.86, p = .016) and a lower prevalence of vertebral (OR = 0.62, 95% CI 0.43-0.91, p = .015) and nonvertebral (OR = 0.51, 95% CI 0.29-0.89, p = .018) fractures. In conclusion, higher serum UA levels are associated with higher BMD at all skeletal sites and with a lower prevalence of vertebral and nonvertebral fractures in older men. © 2011 American Society for Bone and Mineral Research.
Stabilita associazione tra livelli di acido urico e diabete
Livelli sierici elevati di acido urico risulterebbero associati allo sviluppo di diabete di tipo 2. Sono i risultati di un'ampia metanalisi pubblicata da un gruppo di ricercatori giapponesi su Diabetes Care. L'indagine ha preso in considerazione ben 11 studi clinici, presenti nei database Medline (dal 1966 al 2009) ed Embase (dal 1980 al 2009), riguardanti oltre 42mila pazienti diabetici e con follow-up compresi tra 2,0 e 13,5 anni. In sintesi, gli autori hanno calcolato un rischio relativo di sviluppare diabete pari a 1,17 per ogni incremento di 1 mg/dL di acido urico nel siero. "Trattandosi di studi osservazionali non è stato possibile stabilire una relazione causa-effetto tra livelli di acido urico e diabete" ha commentato Hirohito Sone del Department of Internal Medicine, University of Tsukuba di Ibaraki. "Per stabilire se i livelli di questa sostanza possano rappresentare un fattore predittivo dello sviluppo di diabete sarà necessario valutare non solo la suddetta relazione causa-effetto ma anche i valori di cut-off di acido urico". (L.A.).
Diabetes Care 2009, 32, 1737-1742
Serum uric acid is associated with bone health in older men: A cross-sectional population-based study. 2011 J Bone Miner Res. 2011 May;26(5):955-64. doi: 10.1002/jbmr.286.
Serum uric acid (UA) is a strong endogenous antioxidant. Since oxidative stress has been linked to osteoporosis, we examined the association between serum UA levels and bone mineral density (BMD), prevalent vertebral and nonvertebral fractures, and laboratory measures such as calcitropic hormones and bone turnover marker levels. This cross-sectional analysis consisted of 1705 community-dwelling men aged 70 years or over who participated in the baseline part of the Concord Health and Ageing in Men Project (CHAMP), a population-based study of older men in Sydney, Australia. BMD at all sites was significantly higher among men with serum UA levels above the group median than among men with UA levels below the median. In multiple regression analyses adjusted for potential confounders, serum UA remained associated with BMD at all sites (β = 0.12 to 0.14, p < .001), serum calcium (β = 0.11, p = .001), parathyroid hormone (β = 0.09, p = .002), 25-hydroxyvitamin D (β = 0.09, p = .005), and was negatively associated with urinary excretion amino-terminal cross-linked telopeptide of type 1 collagen (β = -0.09, p = .006). Overall, serum UA accounted for 1.0% to 1.44% of the variances in BMD (R(2) = 0.10 to 0.22). In multiple logistic regression analyses, above-median serum UA levels were associated with a lower prevalence of osteoporosis at the femoral neck [odds ratio (OR) = 0.42, 95% confidence interval (CI) 0.22-0.81, p = .010) and lumbar spine (OR = 0.44, 95% CI 0.23-0.86, p = .016) and a lower prevalence of vertebral (OR = 0.62, 95% CI 0.43-0.91, p = .015) and nonvertebral (OR = 0.51, 95% CI 0.29-0.89, p = .018) fractures. In conclusion, higher serum UA levels are associated with higher BMD at all skeletal sites and with a lower prevalence of vertebral and nonvertebral fractures in older men. © 2011 American Society for Bone and Mineral Research.
Working Hypothesis
Metabolite profiling reveals new insights into the regulation of serum urate in humans, 2014