Marianna Gorgellino - Simone Caccia
REWARD SYSTEM

The reward system is a cerebral circuit that gives particular importance to a sensory, emotional or affective stimulus, identified as relevant for the individual wellness and pleasure. Functional Magnetic Resonance Imaging (fMRI) and Positron Emission Tomography (PET) permitted to localize anatomically this circuit: the mesolimbic pathway has a central role, particularly Nucleus Accumbens (NAcc) and Prefrontal Cortex (PFC); its activation is correlated to the intensity of craving.
The biological importance of this neuronal system is demonstrated by the strong conservation observed through different species, from Drosophila Melanogaster, to rats, to humans.
In the mid-brain, Ventral Tegmental Area of Tsai (VTA) is involved in the circuit responsible for reward, and its axonal projections reach both cerebral cortex and Nuclus Accumbens (via the medial fore-brain bundle). The neurotransmitter released in the connections of the mesolimbic pathway is dopamine .
The function of the reward system consists of reinforcing the interest towards what provokes individual pleasure, and all the behaviours and strategies to gain it.
This physiological cerebral function can undergo alterations that can lead to the condition of addiction, a situation in which an exaggerated activation of the circuit caused by a determined input is observed.
Related link:
The Rewarding System
ROLE OF NICOTINE
Among the several sources of activation of the reward system, there is nicotine: in quantitative terms is the most representative form of addiction, habitually used in many countries, even the 50% of population in some industrialized countries and in general one-third of adults worldwide.
Tobacco use causes five million deaths per year, and if the present trend continues, 10 million smokers per year are predicted to die by 2025. Although most smokers wish to stop, few succeed.
Indeed, relapse rates are as high as 80% one year after the quit date, even with the help of the available medications and additional non-pharmacological therapies.
Nicotine dependence is believed to be a complex, polygenically and environmentally determined disorder, indeed recent data suggested that heavy smoking is not only the clinically most relevant, but also the most heritable symptom of nicotine dependence.
Heritability of smoking persistence has been estimated to be at least 50%; moreover twin and adoption studies have suggested that heritability for smoking initiation and smoking persistence is in the range of 59% in males and 46% in females.
In addition, it is now increasingly recognized that nicotine dependence cannot be considered as a unitary phenomenon but that different smokers may smoke for different reasons: alcohol consumption, lifestyle factors, environmental factors, personality traits such as reward dependence and psychiatric disorders are also known to be correlated with nicotine dependence; some of these factors may undergo changes during abstinence, which in turn may lead to relapse.
Related links:
Dramatic decreases in brain reward function during nicotine withdrawal 1998
NICOTINE: Molecular & Physiological Effects in the Central Nervous System
Association study of 45 candidate genes in nicotine dependence in Han Chinese 2012

Nicotine is a natural alkaloid and the principal psychoactive substance found in tobacco. In its uncharged form, nicotine is membrane permeable, therefore, it can influence intracellular processes both indirectly, via nAChRs, and directly by entering the cytoplasm.
Nicotine acts modifying the firing modes and firing frequency of dopaminergic neurons through excitatory and inhibitory inputs and synaptic plasticity.
nAChR
Nicotine is a selective agonist of nicotinic acetylcholine receptor (nAChR); different nAChR subtypes have unique expression patterns in the central nervous system, present also on the dopaminergic neurons of VTA.
Activation of nAChRs occurs when acetylcholine or nicotine binds to the nAChRs, causing a conformational change and opening of the channel pore to allow influx of sodium and calcium.
In neuronal nAChRs, the acetylcholine binding site is located at the α and either γ or δ subunits interface (or between two α subunits in the case of homomeric receptors) in the extracellular domain near the N terminus. When nicotine binds to the site, all the subunits undergo a conformational change, opening a pore with a diameter of about 0.65 nm.
The subsequent activation of receptors modifies the state of neurons through two main mechanisms. On one hand, the movement of cations causes a depolarization of the plasma membrane (which results in an excitatory postsynaptic potential in neurons), but also by the activation of voltage-gated ion channels.
On the other hand, the entry of calcium acts, either directly or indirectly, on different intracellular cascades leading, for example, to the regulation of the activity of some genes or the release of neurotransmitters.
Acting mainly via mid-brain nAChRs composed of the β2 subunit in combination with the α4 and/or α6 subunits, nicotine increases the firing rate and phasic burst activity of mid-brain dopaminergic neurons and elevates dopamine in the PFC, NAcc, and other targets.

A fundamental property of nAChRs is their susceptibility to desensitization and inactivation following, and indeed, in some cases, independent of channel opening.
Prolonged or repeated exposure to a stimulus often results in decreased responsiveness of receptors toward a stimulus, termed desensitization. nAChR function can be modulated by phosphorylation by the activation of second messenger-dependent protein kinases.
PKA and PKC have been shown to phosphorylate the nAChR resulting in its desensitization. It has been reported that, after prolonged receptor exposure to the agonist, the agonist itself causes an agonist-induced conformational change in the receptor, resulting in receptor desensitization.
Desensitized receptors can revert back to a prolonged open state when an agonist is bound in the presence of a positive allosteric modulator.
As a consequence, the low-frequency tonic dopamine release is inhibited , but the phasic dopamine release is regulated differently, such that phasic bursts induce large dopaminergic signals.

While chronic nicotine exposure leads to receptor desensitization, it also leads to compensatory changes such as up-regulation of nicotine binding sites.
Chronic nicotine exposure has been shown to lead to robust nAChR up-regulation in both human smokers and animal models and up-regulation itself is responsible of nicotine’s main effects. The mechanisms invoked for nAChR up-regulation are multiple and likely act in parallel.
As a matter of fact nicotine is not hydrolysed by acetylcholine esterase, as ACh is, so nicotine’s long-lasting presence favours excessive nAChR desensitization and the consequent homoeostatic response to desensitized (turned-off) receptors is up-regulation.
Other mechanisms that likely contribute to up-regulation are decreased surface receptor turnover and increased receptor assembly at the endoplasmic reticulum; isomerization of surface nAChRs to high-affinity nicotinic sites has also been proposed.
Recent electro-physiological studies have shown that both nAChR activation and desensitization contribute to the effects of nicotine in the brain: for instance, desensitization of nAChRs may contribute to the salience of environmental cues associated with smoking behaviour and activation, and desensitization of nAChRs may contribute to both primary and conditioned drug reward (the antidepressant-like effects of nicotinic agents have revealed a balance between activation and desensitization of nAChRs).
Related links:
Acetylcholine Receptors
Nicotinic acetylcholine receptor
Neurophysiology of Nicotine Addiction 2011
Reward, Addiction, Withdrawal to Nicotine 2012
Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system 2011
Nicotine addiction and nicotinic receptors: lessons from genetically modified mice 2010
It's not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood 2009
It has been noticed that nicotine influences the reward circuit even in an indirect way, altering the balance of inputs coming from two types of neurons which participate to the regulation of its state of activation. This additional stimulation increases the pleasure deriving from smoking and its persistence.
Nicotine binding to VTA’s neurons determines only a few minutes stimulation, while dopamine levels in Nucleus Accumbens remain elevated for a longer period.
This temporal difference is explained by the role of GABAergic and glutamatergic neurons: the first ones inhibit the activity of dopaminergic neurons, releasing GABA, while the second ones cause an activation of dopaminergic neurons, through the release of Glu.
In the VTA, nicotine is responsible for the increase of the Glu/GABA ratio, overbalancing the system towards a prolonged activation of the dopaminergic pathway.
Indeed, through exposition of VTA neurons to nicotine for 10 minute (about the time necessary to smoke a cigarette) it was observed a long term potentiation occuring in glutamatergic neurons, causing the prolonged high levels of Glu; on the opposite GABAergic neurons showed an initial increase in GABA transmission lasting only a few minutes, follwed by a decrease and they did not recover fully for more than an hour after nicotine exposure.
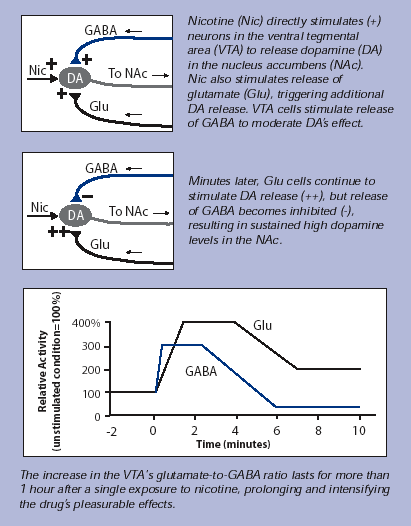
In conclusion, short nicotine exposition, induces permanent changes in the mesolimbic pathway involved in the reward system; these mechanisms are critical for developing addiction.
Nicotine is the psychoactive substance that better and more rapidly induces the processes responsible for addiction.
Related link:
Nicotine’s Multiple Effects on the Brain’s Reward System Drive Addiction 2003