- Farmaco : Tesavel
- Principio Attivo : Sitagliptin Fosfato Monoidrato
- ATC : A10BH01
- Categoria Farmacoterapeutica : Inibitore della DPP-4
- Indicazione Terapeutica : Per Pazienti con Diabete Mellido di Tipo 2 e'
Indicato per :
Migliorare il Controllo Glicemico in Associazione con Metformina Quando
Dieta ed Esercizio Fisico piu' Metformina da Sola Non Forniscono un
Controllo Adeguato della Glicemia.
Migliorare il Controllo Glicemico in Associazione con una Sulfonilurea
Quando Dieta ed Esercizio Fisico piu' la Dose Massima Tollerata di una
Sulfonilurea da Sola Non Forniscono un Controllo Adeguato della Glicemia
e Quando la Metformina Non e' Appropriata per Controindicazioni o
Intolleranza.
Migliorare il Controllo Glicemico in Associazione con una Sulfonilurea e
Metformina Quando Dieta ed Esercizio Fisico piu' la Duplice Terapia con
Questi Farmaci Non Forniscono un Controllo Adeguato della Glicemia
Per Pazienti con Diabete Mellito di Tipo 2 nei Quali e' Appropriato l'Uso
di un Agonista PPARY (cioe' un Tiazolidinedione) e' Indicato per :
In Associazione con l'Agonista PPARY Quando Dieta ed Esercizio Fisico
piu' l'Agonista PPARY da Solo Non Forniscono un Controllo Adeguato
della Glicemia
- Farmaco : Galvus
- Principio Attivo : Vildagliptin
- ATC : A10BH02
- Categoria Farmacoterapeutica : Inibitori della Dipeptidil Peptidasi 4 (DPP-4)
- Indicazione Terapeutica : Trattamento del Diabete Mellito di Tipo 2
In Duplice Terapia Orale in Associazione a:
- Metformina, in Pazienti con Insufficiente Controllo Glicemico Nonostante la
Somministrazione della Dose Massima Tollerata di Metformina in Monoterapia
- Una Sulfanilurea, in Pazienti con Insufficiente Controllo Glicemico Nonostante
la Somministrazione della Dose Massima Tollerata di una Sulfanilurea e per i
Quali la Terapia con Metformina e' Inappropriata a Causa di Controindicazioni
o Intolleranza
- Un Tiazolidinedione, in Pazienti con Insufficiente Controllo Glicemico e per i
quali e' Appropriato l'Uso di un Tiazolidinedione
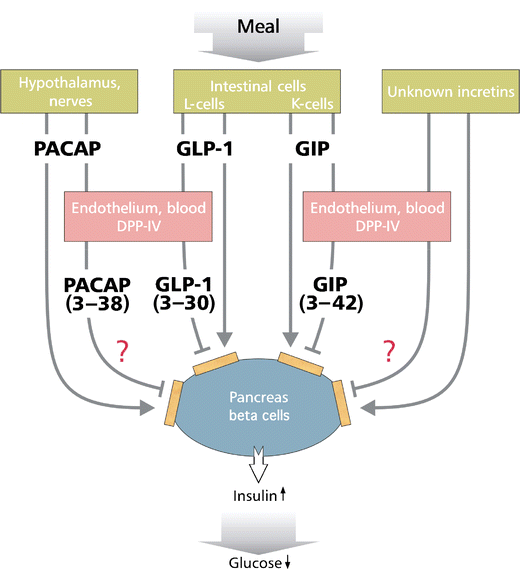
DPP-IV multiple roles, 2000

Dipeptidyl peptidase inhibitors as new drugs for the treatment of type 2 diabetes
OMIM
OMIM Gene Map
Side effects: CD26 and immunodeficiency
some papers.......