Cavinato Andrea
Bellecca Riccardo
A neurexin (NRXN) is a presynaptic protein that helps to glue together neurons at the synapse. Neurexins are type I membrane proteins that can be classified into two types, α-NRXNs and β-NRXNs.
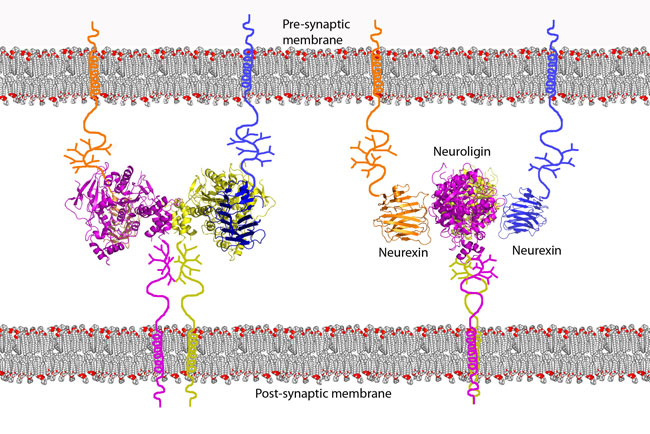
They are encoded by several unlinked genes of which two, NRXN1 and NRXN3, are among the largest known human genes. Three of the genes (NRXN1-3) utilize two alternate promoters and include numerous alternatively spliced exons to generate thousands of distinct mRNA transcripts and protein isoforms. Neurexins mediate signalling across the synapse, and affect the properties of neural networks by specifying synaptic functions. Neurexins and neuroligins are core components of the molecular machinery that controls synaptic transmission and enables neural networks to process complex signals. In humans, alterations in genes encoding neurexins are implicated in autism and other cognitive diseases, in drug, tobacco and alcohol addiction and play and important role in angiogenesis.
Association of Neurexin 3 polymorphisms with smoking behaviour
Neurexin-3-alpha is a protein that in humans is encoded by the NRXN3 gene. NRXN3 is an interesting gene in relation to addiction vulnerability for a number of reasons. Its expression in both development and in adult brain, its likely contributions to proper development and maintenance of excitatory and inhibitory synapses and its expression in brain circuits important for reward and memory all provide highly interesting contexts for potential NRXN3 roles in altering vulnerability to addictions. NRXN3 is located on chromosome 14 and the two existing promoters drive the expression of its α and β isoforms. Together with five canonical sites of alternative splicing, these promoters give rise to over 1000 isoforms.
Studies ofNRXN3 gene's structure and variants provide numerous sites at which variation could influence NRXN3 function, brain structure, brain function and behaviour.
Tobacco use continues to be the leading global cause of preventable death. It kills nearly 6 million people every year through cancer, heart disease, respiratory diseases, childhood diseases and others. It also causes hundreds of billions of dollars of economic losses worldwide every year. Over the course of the 21st century, tobacco use could kill up to a billion people. (WHO 2012)
The development of nicotine addiction is influenced by environmental and genetic factors, and while environmental factors have a stronger influence on initiation, genetic factors play a more significant role in the transition from regular use to addiction. A genetic component in the development of nicotine addiction has been showed in twin and family studies, showing an estimated heritability of 30–72%. Twin studies (Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data, 2002) also showed a hereditary component for a range of diverse smoking-related phenotypes, including age at initiation, intensity and cessation. Similarly, familial studies showed that siblings of habitual-smoking probands are at greater risk of becoming habitual smokers.
To evaluate the role of NRXN3 in nicotine addiction, single nucleotide polymorphisms (SNPs) and copy number variant (CNV) have recently been analyzed within the NRXN3 genomic region, showing that three SNPs were significantly associated with a lower risk of being a smoker odds ratio while there was no association between the CNV polymorphisms and smoking behaviour. There’s no relationship between these SNPs and the level of addiction, as determined by the Fagerström index.

Notably, NRXN3 SNPs associated with smoking behaviour are located in an intronic region and no specific effect was predicted for either SNP. Although this intronic region is highly conserved between species, suggesting that it fulfils an important role.
Several of the SNPs included in this work have also been analyzed in other studies , although most of them did not show any association, one of them had been associated with heavy smoking in schizophrenic patients and has shown nominal association with alcohol problems in men (Associations among types of impulsivity, substance use problems and Neurexin-3 polymorphisms, 2011), and another one had been associated with alcohol dependence (Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms, 2007).
Considering that in the three mentioned studies were been recruited samples from different populations, the lack of association between most of the SNPs can be due to genetic heterogeneity among populations or may reflect distinct gene per environment interactions.
Conclusions
Starting from this work, we can notice that, while most of the neurological and pharmacological fundament concerning addictive behaviours are known, there are a lot of new discoveries to be done if we consider the genetic aspect of this phenomena.
Neurexin involvement in visual function
A brand new study (Neurexin Regulates Visual Function via Mediating Retinoid Transport to Promote Rhodopsin Maturation, 2013) has assessed important relationships between neurexins and visual function. In this is shown that the Drosophila homolog of α-Neurexin is critical for fly visual function. Lack of Neurexin leads to significantly impaired visual function due to reduced rhodopsin levels.
It has been revealed that Neurexin mediates Rh1 maturation through regulating retinoid transport, which is essential for rhodopsin maturation and further demonstrated that the intracellular region of Neurexin interacts with apolipoprotein I (ApoL I) and is required for the stability of ApoL I and II, key proteins that function in transporting retinoids in the retina. The results reveal a role for Neurexin in mediating retinoid transport and subsequent rhodopsin maturation and suggest that Neurexin regulates lipoprotein function.

Membrane receptors are responsible for translating extracellular stimuli into intracellular responses. The successful intracellular transport of rhodopsin to light sensory organelles is essential for photoreceptor function and survival, as defects in rhodopsin transport lead to severe retinal degeneration. Loss of Neurexin leads to reduced Rh1 levels and impaired visual function. Eye-specific expression of Neurexin rescues the impaired Rh1 level and visual function in the mutant (knockout for some neurexins genes). The compelling evidence that the cell adhesion molecule is required for rhodopsin maturation and function.

Neurexin is localized in the rhabdomeres in photoreceptors and photoreceptor-specific expression of Neurexin is able to rescue the impaired Rh1 level in the mutant. These results reveal that photoreceptor-derived Neurexin is essential for Rh1 maturation. The canonical binding partners of Neurexins, Neuroligins, are thought to be important for establishing the asymmetry of the synapses. However, unlike with Neurexin, loss of Neuroligin did not alter Rh1 levels. Taken together, the results suggest that Neurexin is probably activating via a Neuroligin and synapse-independent manner to regulated Rh1 maturation in the eye.
Neurexin has also an important role in retinoid transport. The experiment has been made on Drosophila, considering that it is a good model system for genetic and molecular studies of vitamin A metabolism, because vitamin A is not required for fly viability but is critical for the generation of chromophores and for the synthesis of visual pigments.
In carotenoid-deprived mutants of Drosophila, defective chromophore production observed outside the retina can be rescued by supplying vitamin A in food. However, is unsuccessful to restore Rh1 levels in mutants by supplying all-trans retinal in food. In contrast, it’s possible to restore Rh1 levels by expressing Neurexin or RFABG in the photoreceptors. These results further support the conclusion that Neurexin function inside the retina is to facilitate chromophore generation or transport.
Neurexin interacts with ApoL protein
Neurexins are single-pass transmembrane proteins and the intracellular domain of Neurexin interacts with a number of exocytotic proteins, such as Velis, Munc18, and CASK.
The expression of the intracellular region of Neurexin is sufficient to restore the Rh1 level. There are evidences that the ApoL protein levels, which are reduced in mutant retina, can be restored by an overexpression of Neurexin. Some results (Interphotoreceptor Retinoid-Binding Protein (IRBP) is Rapidly Cleared From the Xenopus Interphotoreceptor Matrix, 1999) provide evidence that the intracellular region of Neurexin plays an important role in stabilizing ApoL proteins.

Importance of the discovery
In this study, it’s shown that ApoL protein levels are reduced in mutant retina and Rh1 levels are restored upon overexpression of RFABG in the mutant eye. Sustained overexpression of RFABG might compensate the reduced stability of ApoL proteins in the mutant eye. These observations are consistent with the Neurexin rescue experiments, which show expression of Neurexin in photoreceptors is sufficient for restoring Rh1 level in the mutant.
Nutritional and environmental factors play important roles in ASD, and fatty acid metabolism and abnormal membrane fatty acid composition may contribute to this disorder. It has been reported that Apo lipoproteins, especially Apo B-100, are reduced in children with ASD. Drosophila RFABG show high similarity in its domain structure with vertebrate Apo B-100. The region aa 1,390–1,480 of RFABG is sufficient for the interaction with Neurexin. The sequence aa 1,390–1,480 is Lysine enriched.These highly charged residues could be important in mediating the Neurexin/ApoL interaction. In addition, this region is conserved between Drosophila RFABG and vertebrate IRBP and Apo B-100, implying that the Neurexin/ApoL I interaction may be conserved among various species. The revelation about interaction and function correlation between Neurexin and lipoproteins may have put a step forward for us to understand pathological relations of Neurexin mutations and perturbed fatty acid metabolism in ASD patients.