Introduction
Skin aging is a multisystem degenerative process that involves the skin and the skin support system.
Skin aging may be caused by several factors, such as, UV irradiation, stress, or smoking.
Acute exposure of human skin to UV irradiation causes sunburn, inflammation, immune suppression, and dermal connective tissue damage, and chronic exposure to UV over many years disrupts the normal skin architecture and ultimately causes photoaging and even skin cancer.
Wrinkle formation is a striking feature of photoaged skin and is caused by the degradation of collagen fibrils and gelatin fibers.
UV increased release of pro-inflammatory mediators from a variety of skin cells, resulting in MMP activation. However, inflammation activated various matrix-degrading MMP, which leads to abnormal matrix degradation and accumulation of non-functional matrix components. In addition, reactions to UV-induced inflammation in skin were enhanced in aging skin, such as deep wrinkles and thickening of the dermis and epidermis.
UV damages de novo type І collagen synthesis, and UV-induced AP-1 downregulates type І collagen, the most abundant protein in skin connective tissue. This process, called collagen degradation, results in the expressions of MMPs (Wikipedia), which are responsible for wrinkle formation in photodamaged skin.
AP-1 tightly regulates the transcriptions of several MMPs (matrix-metalloproteinase).
Garlic ( Active Garlic Components , September 2013) has several effects, it acts as an antioxidant, inhibits NF-κB activation, and protects against UV-induced immunity suppression. Caffeic acid (CA) is found in garlic, fruits, and coffee contains both phenolic and acrylic functional groups. CA is a well-known pharmacological antioxidant with antimutagenic activities and anti-inflammatory and immunomodulatory effects. Some studies have also shown that carcinogenesis is inhibited by CA. CA is well-known for possible anti-wrinkle agent. CA suppressed UVB-induced photoaging by inhibiting MMPs and elevating type І procollagen production through ROS scavenging and down-regulation of MAP kinases pathway.
S-allyl cysteine (SAC) is the most abundant compound in AGE (aged garlic extract). Uracil is also present in garlic, and has a well-known anticancer effect and increases cell viability.

Structures of the three active garlic compounds.
(A) Caffeic acid,(B) S-allyl cysteine,© Uracil.
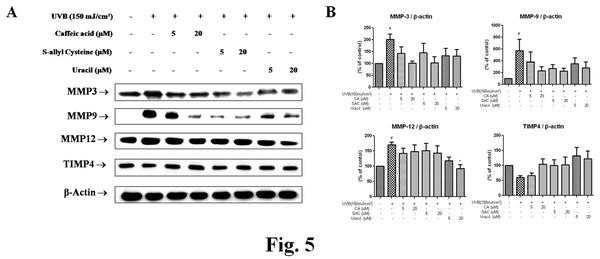
Modulation of UVB-induced MMPs by the active compounds from garlic
MMP induction is, in part, responsible for UV-induced damage to skin connective tissues. MMPs have the ability to degrade mature fibrillar collagen completely. In addition to impairing collagen synthesis, increased levels of several MMP family members, occurs in chronologically aged skin. These observations are consistent with induction of transcription factors NF-κB and AP-1 by UVB irradiation.
As shown in Figure 5A, levels of MMPs protein were elevated in UVB treated groups but pretreatment with CA, SAC, or uracil suppressed these levels. These results suggest that the three active compounds protect skin connective tissue from UVB via the suppression of MMPs and TIMP4 degradation.
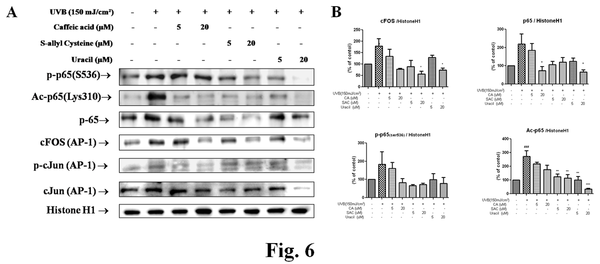
Effects of active compounds from garlic on MMPs-related transcription factor during photoaging
The protein levels of NF-κB family, including p-p65, p65, and acetyl-p65, were increased by UVB but decreased by the three garlic compounds. Likewise, these compounds also significantly decreased cFOS and p-cJun (components of AP-1) levels (Figure 6).
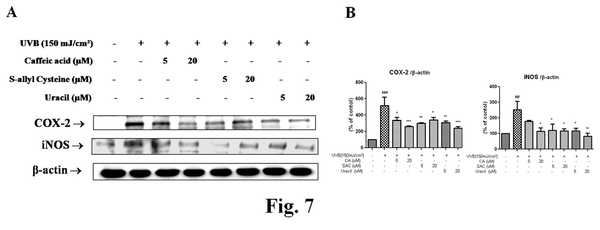
Active compounds from garlic inhibited the expressions of NF-κB-dependant genes
COX-2 and iNOS are genes known to be associated with inflammation and to possess an NF-κB binding site in their promoter regions. As shown in Figure 7, COX-2, and iNOS protein levels were increased by UVB, but pretreatments with CA, SAC, and uracil decreased these increases (Figure 6). These results suggest that CA, SAC, and uracil modulate NF-κB activation and the expressions of NF-κB-dependant genes.
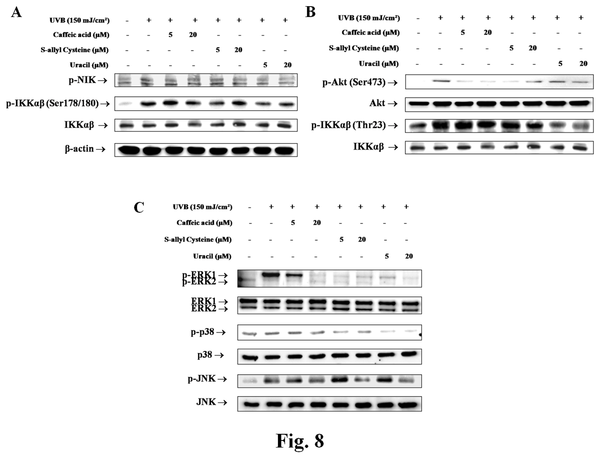
Changes in NF-κB signaling pathway by active compounds from garlic
Akt activation was significantly increased by UVB, but pretreatment with CA, SAC, and uracil reduced these activations. Levels of phospho-IKKαβ and phospho-IKKαβ were also considerably decreased, and phospho-NIK, the upstream gene of phospho-IKKαβ in hairless mice, was strongly downregulated all three compounds (Figure 8A and 8B). In addition, MAPK phosphorylation was detected using antibodies for p-ERK1/2, p-JNK, and p-p38. The protein levels of all three were increased by UVB skin, but CA, SAC, and uracil pretreatments decreased these increases (Figure 8C).
Inhibitory effects of the three compounds on UVB-induced oxidative stress
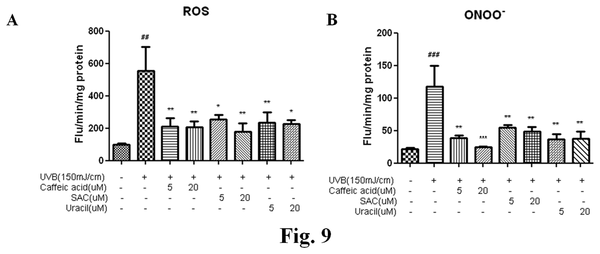
Oxidative stress is a primary factor in the photoaging process. UVB increases levels of hydrogen peroxides and other reactive oxygen species in skin and decreases the levels of anti-oxidant enzymes. These features are also observed in chronologically aged human skin. In both cases, increased ROS(Wikipedia) production alters gene and protein structures and functions and ultimately leads to skin damage. ROS are necessary participants in multiple MAP kinase pathways, and MAPK activation results in the inductions of NF-κB and/or AP-1, which, in turn, upregulate the expressions of MMPs. This cascade provides a mechanism for the increased collagen degradation observed in photoaged skin.
The increased levels of ROS post-UVB irradiation were found to be suppressed by pretreatment with CA, SAC, or uracil in a dose-dependent manner (Figure 9A). Furthermore, ONOO¯ levels were higher in UVB-irradiated group compared to untreated group, showing that CA, SAC, and uracil pretreatments inhibited ONOO¯ generation (Figure 9B).
Discussion
Photoaging is the result of the superposition of repetitive ultraviolet (UV)-induced damage and intrinsic aging and accounts for most age-associated changes in skin. Photoaging is triggered by receptor-initiated signaling, mitochondrial damage, protein oxidation, and telomere-based DNA damage responses. UV-irradiated skin displays variable epidermal thickness, increased collagen fragmentation, increased levels of matrix-degrading metalloproteinases, dermal elastosis, inflammation, and vessel ectasia. In addition, wrinkle formation is a striking feature of photoaged skin and is caused by the degradation of collagen fibrils and gelatin fibers.
UVB irradiation increases wrinkle formation and collagen disorder. Although these data clearly demonstrated the anti-wrinkle effect of active garlic compounds in vivo.
The most significant cause of photodamage is oxidative stress, which is considered to play a central role in initiating and driving the signaling events that lead to cellular responses to UV exposure. Furthermore, UV irradiation increase levels of hydrogen peroxide and ther reactive oxygen species (ROS), and reduce levels of anti-oxidant enzymes. CA and SAC inhibit oxidative stress. Uracil was able to lower ROS and peroxynitrite levels significantly in UVB-irradiated hairless mice (Figure 9).
Major signaling pathways known to mediate UVB-induced biological responses involve mitogen-activated protein kinases (MAPKs). MAPKs mediate a wide range of intracellular signaling molecules that are involved in processes, such as, inflammation, cell proliferation, differentiation, and apoptosis. Three types of MAPKs have been characterized, that is, the extracellular signal-regulated kinases (ERKs), the c-Jun N-terminal kinases (JNKs), and p38. The phosphatidylinositol-3 kinase (PI-3K) pathway is another key activator of UVB-induced cellular responses. PI-3K is a major kinase upstream of Akt( Wikipedia), and the PI-3K pathway regulates various cellular processes, such as, apoptosis, proliferation, and growth, and requires UVB-induced NF-κB activation via the phosphorylation of IKKαβ (Thr23). CA decreased UVB-induced MMP expression and ROS formation (Figure 5). Increased ROS levels act to amplify the signal leading to the activations of MAPKs, Akt, and IKKαβ.
UVB-induced cellular damage at the DNA and molecular levels initiates the activations of transcription factor pathways, which in turn regulate the expressions of a number of genes, such as, COX-2, MMPs, TNFα, and IL-1. Nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) are two representative transcription factor families. NF-κB regulation can also be influenced by signaling cascades that are involved in the activation of AP-1. Transcription factors, such as, NF-κB and AP-1, are influenced by active garlic compounds (Figure 6).
The level of nuclear p65 decreased in a dose-dependent manner in vivo after treatment with CA, SAC, or uracil. Furthermore, these compounds downregulated cFOS levels, which were significantly increased by UVB (Figure 6).
The increased activities of AP-1 and NF-κB downregulate type I procollagen and TIMP (tissue inhibitor of metalloproteinases) and upregulates MMPs. TIMP4 is the newest member of the mammalian TIMP family. The expressions of these genes are remarkably reduced by UVB irradiation and aging, whereas UVB increases the synthesis of matrix metalloproteinases (MMPs). MMPs are responsible for degradation of ECM, cause collagen and elastin degradation. In the present study, levels of MMPs were increased by UVB and these increases were downregulated by CA, SAC, or uracil pretreatment in vivo. In addition, decreases in type I procollagen levels were prevented by pretreatment with CA, SAC, or uracil, which suggests their potential uses as wrinkle suppressants. Furthermore, it is remarkable that uracil effectively inhibited the inductions of MMP gene expressions by UVB and procollagen production.
Summarizing, the three active garlic compounds effectively inhibited wrinkle formation induced by UVB irradiation, and this inhibition was attributed to increased type I procollagen production via the downregulation of MMPs and upregulation of TIMP4. Wrinkle formation was also reduced by the CA, SAC, and uracil (Figure 1), which inhibited oxidative stress as follows: 1) CA and SAC decreased oxidative stress by directly affecting scavenging and modulating NF-κB or AP-1. 2) CA, SAC, and uracil exhibited anti-inflammatory effects via the suppressions of COX-2 and iNOS. (Figure 10).

Mechanism of NF-κB action.

In an inactivated state, NF-κB ( Wikipedia ) is located in the cytosol complexed with the inhibitory protein IκBα. Through the intermediacy of integral membrane receptors, a variety of extracellular signals can activate the enzyme IκB kinase (IKK). IKK, in turn, phosphorylates the IκBα protein, which results in ubiquitination, dissociation of IκBα from NF-κB, and eventual degradation of IκBα by the proteosome. The activated NF-κB is then translocated into the nucleus where it binds to specific sequences of DNA called response elements (RE). The DNA/NF-κB complex then recruits other proteins such as coactivators and RNA polymerase, which transcribe downstream DNA into mRNA, which, in turn, is translated into protein, which results in a change of cell function.
Summary of mechanism previously explained
