Grinza Marta
Giolitti Francesca
Introduction
A multidisciplinary study demonstrated the ability of a component of snake venom to inhibit cancer cell migration by binding to a cell-surface protein in the integrin family, preventing it gripping the extracellular matrix, by scrambling signals to the cytoskeleton and by interacting with angiogenic processes. The protein, called contortrostatin, was discovered into the venom of the Agkistrodon contortrix contortrix by Francis S. Markland, Ph.D, a professor of biochemistry and molecular biology at the University of Southern California Keck School of Medicine, Los Angeles.
The snake
Agkistrodon contortrix contortrix is a species of venomous snake endemic to Nord America, a member of the Crotalinae (pit viper) subfamily. The common name for the species is the southern copperhead.

The venom of snakes is a modification of saliva and it is produced by parotid salivary glands, usually situated on each side of the head, below and behind the eye and encapsulated in a muscular sheath.
Venoms contain more than 20 different compounds, mostly proteins and polypeptides. This complex mixture of proteins, enzymes, and various other substances has toxic and lethal properties. Some of the proteins have very specific effects on various biological functions and have also been developed for use as pharmacological or diagnostic tools or even useful drugs, as in this case.

Discovery
Markland was examining venom from the southern copperhead for its clot-busting properties when he learned that a group in Taiwan had found disintegrins, integrin antagonists, in the venom of another snake and he thought it would be interesting to see if a similar protein was present in southern copperhead venom. After purifying the venom by means of multiple step high performance liquid chromatography, Markland and his colleagues found a minor component of the venom that worked as a disintegrin, and this is the protein they named contortrostatin. They knew on the basis of contortrostatin’s ability to inhibit platelet aggregation that it was interacting with integrins, and they also knew that there are integrins on the surface of cancer cells. Therefore they reasoned that it might be fruitful to look at its anticancer activity.
(Snake Venom Protein Paralyzes Cancer Cells, Journal of the National Cancer Institute; 2001)
Integrins and disintregrins

Contortrostatin is a 13.5 kDa homodimeric disintegrin that function as potent inhibitors of both platelet aggregation, by blockage of fibrinogen receptor αIIbβ3, and integrin-dependent cell adhesion. Disintegrins are small soluble disulfide-rich proteins in which each chain contains 65 amino acids with an Arginine-Glycine-Aspartic acid (RGD) or Lysine-Glycine-Aspartic acid (KGD) sequences near their carboxyl terminus.
These motifs modulate the interaction with integrins on tumor cells and angiogenic vascular endothelial cells.
The integrins are a family of transmembrane receptor proteins, usually present on the surface of normal and tumor cells, that bind to components of the extracellular matrix (in particular integrins bind ligands as fibronectin, vibrinectin, collagen and laminin) and participate in large protein complexes known as focal adhesions (FA).
Integrins have two main functions:
- Attachment of the cell to the extracellular matrix (in this way extracellular matrix will be connected with intracellular actin filamentous system)
- Signal transduction from the extracellular matrix to the cell
Signaling also operates in the opposite direction: signals generated inside the cell can either enhance or inhibit the ability of integrins to bind to their ligand outside the cell.
They are responsible for the motility and they pass information about the chemical composition and mechanical status of the extracellular matrix into the cell.
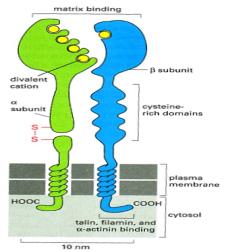
Integrins are obligate heterodimers containing two dinstinct chains called α and β subunits.
Disintegrins interact with the β1 and β3 families of integrins. The binding of integrins to their ligands depends on extracellular divalent cations (Ca2+ or Mg2+) reflecting the presence of divalent-cation-binding domains in the extracellular part of the α and β subunits. The type of divalent cation can influence both the affinity and the specificity of the binding of an integrin to its ligands.
Researchers in a number of laboratories are focusing on one integrin in particular, called αvβ3. This integrin is present on the surface of cancer cells and is thought to be critical in metastasis.
In fact, the metastatic spread of cancer is a complex process that involves a combination of different cellular actions including cell adhesion to the extracellular matrix, breakdown of the extracellular matrix by specific matrix-degrading proteinases, and active cell locomotion.
Normal integrin activity
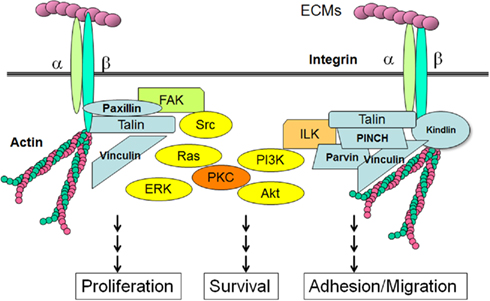
Many of the signaling functions of integrins depend on a cytoplasmic protein tyrosine kinase called
focal adhesion kinase (FAK). Focal adhesions are often the most prominent sites of tyrosine phosphorylation in cells in culture, and
FAK is one of the major tyrosine-phosphorylated proteins found in focal adhesions. When integrins cluster at sites of cell-matrix contact,
FAK is recruited to focal adhesions by intracellular anchor proteins, such as
talin, which binds to the integrin β subunit, or
paxillin, which binds to one type of integrin α subunit. The clustered
FAK molecules cross-phosphorylate each other on a specific tyrosine, creating a phosphotyrosine docking site for members of the
Src family of cytoplasmic tyrosine kinases. These kinases then phosphorylate
FAK on additional tyrosines, creating docking sites for a variety of intracellular signaling proteins; they also phosphorylate other proteins at focal adhesions. In this way, the signal is relayed into the cell.
Contortrostatin activity on integrin
Disintegrins are capable of inhibiting several aspects of tumor cell behavior both in vitro and in vivo, including adhesion, migration, invasion, metastasis and angiogenesis.
Infact, contortrostatin binds to integrins αIIbβ3, α5β1, and αvβ3 and this link induces integrin-mediated tyrosine phosphorylation events and causes severe disruptions in the actin cytoskeleton and disassembly of focal adhesion structures without affecting cellular adhesion to a reconstituted basement membrane. Included in this disruption is the tyrosine phosphorylation and altered subcellular localization of FAK.
In the same way, the linkage between integrins on the cell surface of vessels and contortrostatin, inhibit angiogenesis.
The term angiogenesis usually defines the growth of sprouts from capillary blood vessels that depends on a delicate balance between pro- and anti-angiogenic factors. Angiogenesis is also influenced by integrins that process signals from their microenvironment and respond by altering their cell-cell and cell-matrix adhesion, which allows migration and vascular remodelling.
(Snake Venom Disintegrins and Cell Migration; 2010)
In vivo and in vitro applications:
A great number of studies was lead to show the functionality of contortrostatin in tumors and the majority of them had positive results. Therefore it is possible to consider contortrostatin a real and potent inhibitor of cancer.
Here we mention some of these studies:
- Malignant gliomas are not curable because of diffuse brain invasion. Tumor cells invade the surrounding brain tissue without a clear tumor-brain demarcation line, making complete resection impossible. Reaserchers examined the effect of contortrostatin on glioma progression in a rodent model. Athymic mice were intracranially or subcutaneously injected with U87 glioma cells, and the effect of intratumorally administered contortrostatin on tumor progression and animal survival was then studied. The results demonstrate that contortrostatin is able to inhibit tumor growth and angiogenesis and to prolong survival in a rodent glioma model. Moreover, contortrostatin appears to be well tolerated by the animal and lacks obvious neurotoxic side effects.
(The role of contortrostatin, a snake venom disintegrin, in the inhibition of tumor progression and prolongation of survival in a rodent glioma model; 2005)
- OVCAR-5 is a human epithelial carcinoma cell line of the ovary, established from the ascitic fluid of a patient with progressive ovarian adenocarcinoma without prior cytotoxic treatment. Contortrostatin effectively blocks the adhesion of OVCAR-5 cells to several extracellular matrix proteins and inhibits tumor cell invasion through an artificial basement membrane. In a xenograft nude mouse model with intraperitoneal introduction of OVCAR-5 cells, intraperitoneal injection of Contortrostatin was used for therapy. Contortrostatin not only significantly inhibited ovarian cancer dissemination in the nude mouse model, but it also dramatically prevented the recruitment of blood vessels to tumors at secondary sites.
(Contortrostatin, a homodimeric disintegrin, binds to integrin alphavbeta5; 2000)
- Studies on human breast cancer demonstrate that contortrostatin binds to integrins and blocks the adhesion of tumoral cells (MDA-MB-435) to extracellular matrix proteins including fibronectin and vitronectin, but it has no effect on adhesion of the cells to laminin and Matrigel. Daily local injection of contortrostatin (5 microg per mouse per day) into MDA-MB-435 tumor masses in an orthotopic xenograft nude mouse model inhibits growth of the tumor by 74% ( p = 0.0164 ). More importantly, it reduces the number of pulmonary macro-metastasis of the breast cancer by 68% ( p < 0.001 ), and micro-metastasis by 62.4% ( p < 0.001 ).
(Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits breast cancer progression; 2000)
Conclusions
As we said cell adhesion and migration are crucial steps for metastasis development, processes in which the integrins are strongly involved. Snake venom contortrostatin has been shown to inhibit metastasis by effectively blocking integrin activities.
Not only contortrostatin was founded usefull against cancer, but there are a lot of different disintegrins of other snake venom with the same aim.
Some examples could be:
- Triflavin from Trimeresurus flavoviridis venom was one of the first RGD-disintegrins shown to inhibit angiogenesis both in vitro and in vivo.
- Similar results were obtained with another RGD-disintegrin, rhodostomin, from Agkistrodon rhodostoma venom, which inhibits endothelial cell migration, invasion and tube formation evoked by bFGF in matrigel both in vitro and in vitro. Rhodostomin effects were inhibited by anti- αvβ3 but not by anti- αvβ5 antibodies.
- A monomeric recombinant RGD-disintegrin from Bothrops alternatus venom, DisBa-01, produces similar effects in the matrigel plug model in nude mice. In addition to inhibition of EC migration, saxatilin, an RGD disintegrin from Gloydius saxatilis also inhibited the migration of vitronectin-induced smooth muscle cells.
Contortrostain discovery was a great pace toward the defeat of cancer.
We hope that in the future research will find new paths due to improve the quality of life and find the best way to completely fight cancer and other diseases.