| Author: Maria Cristina Bindolo |

In this report I want to show the correlation between Helicobacter Pylori infection and Pre-eclampsia.
Introduction
The discovery of Helicobacter pylori infection helped the understanding of the natural history of many disorders of the upper gastrointestinal tract, including chronic gastritis, peptic ulcer disease, gastric cancer and MALT lymphoma. Interestingly, epidemiological studies have also revealed a correlation between H. pylori infection and some diseases localized outside the stomach, especially those characterized by persistent and low-grade systemic inflammation. Of note, H. pylori has an important role in iron deficiency anaemia, idiopathic thrombocytopenic purpura, vitamin B12 deficiency and pre-eclampsia. Moreover, the association of this bacterial pathogen with many other diseases, including hepatobiliary, pancreatic, cardiovascular and neurodegenerative disorders is currently under investigation.
Helicobacter Pylori
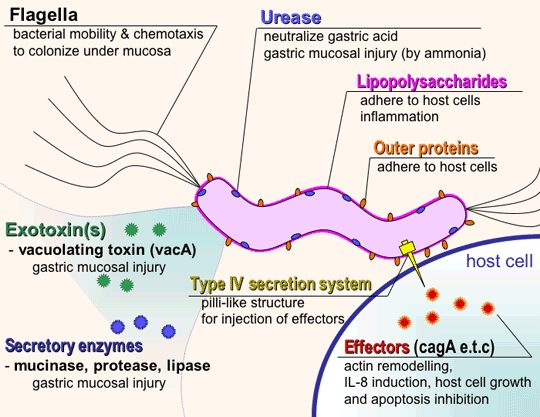
Helicobacter pylori, previously named Campylobacter pylori, is a Gram-negative, microaerophilic bacterium found in the stomach (it requires oxygen, but at lower concentration than it is found in the atmosphere). It was identified in 1982 by Australian scientists Barry Marshall and Robin Warren, who found that it was present in patients with chronic gastritis and gastric ulcers, conditions that were not previously believed to have a microbial cause. It is also linked to the development of duodenal ulcers and stomach cancer. H. pylori has also been associated with colorectal polyps and colorectal cancer. However, over 80 percent of individuals infected with the bacterium are asymptomatic and it has been postulated that it may play an important role in the natural stomach ecology. More than 50% of the world's population harbor H. pylori in their upper gastrointestinal tract. Infection is more prevalent in developing countries and incidence is decreasing in Western countries. H. pylori's helix shape (from which the generic name is derived) is thought to have evolved to penetrate the mucoid lining of the stomach.
H. pylori is a helix-shaped bacterium about 3 micrometres long with a diameter of about 0.5 micrometres. It contains a hydrogenase which can be used to obtain energy by oxidizing molecular hydrogen (H2) produced by intestinal bacteria. It produces oxidase, catalase, and urease. Once H. pylori is safely ensconced in the mucus, it is able to fight the stomach acid that does reach it with an enzyme it possesses called urease. Urease converts urea, of which there is an abundant supply in the stomach (from saliva and gastric juices), into bicarbonate and ammonia, which are strong bases. This creates a cloud of acid neutralizing chemicals around the H. pylori, protecting it from the acid in the stomach. Another defense H. pylori has is that the body's natural defenses cannot reach the bacterium in the mucus lining of the stomach. The immune system will respond to an H. pylori infection by sending white cells, killer T cells, and other infection fighting agents. However, these potential H. pylori eradicators cannot reach the infection, because they cannot easily get through stomach lining. They do not go away either, though, and the immune response grows and grows. Extra nutrients are sent to reinforce the white cells, and the H. pylori can feed on this.
H. pylori consists of a large diversity of strains, and the genomes of three have been completely sequenced. Two of sequenced strains have an approximately 40-kb-long Cag pathogenicity island (a common gene sequence believed responsible for pathogenesis) that contains over 40 genes. This pathogenicity island is usually absent from H. pylori strains isolated from humans who are carriers of H. pylori but remain asymptomatic. The cagA gene codes for one of the major H. pylori virulence proteins. Bacterial strains that have the cagA gene are associated with an ability to cause ulcers. The cagA gene codes for a relatively long (1186 amino acid) protein. The cag pathogenicity island (PAI) has about 30 genes, part of which code for a complex type IV secretion system.
Pre-Eclampsia
Pre-eclampsia is a disorder of pregnancy characterized by high body pressure and large amounts of protein in the urine. Pre-eclampsia is diagnosed when a pregnant woman develops:
• Blood pressure ≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic on two separate readings taken at least four to six hours apart after 20 weeks gestation in an individual with previously normal blood pressure
• Proteinuria ≥ 0.3 grams (300 mg) or more of protein in a 24-hour urine sample
The syndrome is almost certainly the result of multiple factors. It is now thought that abnormal placentation (development and arrangement of the placenta) and placental function is a strong predisposing factor for preeclampsia, though there are contributing and related factors that complicate finding a precise mechanism for preeclampsia. Central to the effects of preeclampsia are the resulting presence of uteroplacental hypoxia (inadequate oxygen supply), oxidative stress, maternal endothelial (lining of blood vessels) dysfunction, and elevated systemic inflammation. This abnormally implanted placenta is thought to result in poor uterine and placental perfusion, yielding a state of hypoxia and increased oxidative stress and the release of anti-angiogenic proteins into the maternal plasma along with inflammatory mediators. The abnormal implantation is thought to stem from the maternal immune system's response to the placenta and suggests a lack of established immunological tolerance in pregnancy. Endothelial dysfunction results in hypertension and many of the other symptoms and complications associated with preeclampsia such as the presence of a low blood platelet count (thrombocytopenia), impaired liver function, the development of new kidney dysfunction, fluid accumulation in the lungs (pulmonary edema), and new-onset brain or visual disturbances. If left untreated, preeclampsia can develop into eclampsia.
It affects 2–7% of all pregnancies and remains one of the major causes of maternal and fetal mortality. It may develop after 20 weeks of gestation, though most commonly after 32 weeks. Preeclampsia occurring before 32 weeks is considered early-onset and is associated with increased morbidity. Maternal mortality attributable to PE has been reduced in developed countries; however, perinatal mortality and neurological sequelae resulting from fetal growth restriction and/or preterm delivery are still unresolved. Moreover, it has been shown that PE mothers are at increased risk for ischaemic heart disease and death from cardiovascular causes later in life. Given the syndromic and mulitfactorial nature of the disease, it is not yet possible to routinely predict preeclampsia. Delivery of the fetus and placenta is the only known treatment for preeclampsia. Rarely, preeclampsia may also occur in the postpartum period.
Correlation between pre-eclampsia and H. pylori
The disappointing therapeutic results reflect the fact that the aetiology and pathogenesis of PE are largely unknown. The most likely hypothesis is that a generalized endothelial dysfunction is the first step in the development of PE. It has been proposed that in PE, the normal inflammatory response of pregnancy, involving intravascular leucocytes and the clotting system, is exaggerated. There is mounting evidence that certain infectious agents can induce endothelial inflammation and injury; the bacterium Helicobacter pylori is one of these agents. It has been demonstrated that this pathogen enhances platelet activation and therefore thrombus formation.

Simona Cardaropoli, from the University of Turin, and collegues investigated in 2006 the prevalence of seropositivity for IgG against H. pylori and cytotoxin-associated antigen A (CagA) in PE patients and the presence of H. pylori DNA in their placentas. In addition, they searched for the presence of H. pylori DNA in the placentas from normal and PE mothers. In PE women, there were significantly more H. pylori seropositive subjects (51.1%) than in normal pregnant women (31.9%). Anti-CagA antibodies were significantly more common among PE mothers (80.9%) than among normal pregnant women (14.9%).
To better understand the role of the infection in the pathogenesis of PE, they further looked for the presence of H. pylori DNA in the placenta. All the placentas were negative, thus indicating that the pathogenic mechanism is not a local one. This finding, along with the knowledge that H. pylori is predominantly acquired during childhood and that the infection is lifelong when untreated, elicits the speculation that H. pylori positive women may have underlying vascular damage; such subclinical dysfunction might augment the inflammatory changes of pregnancy, thus contributing to the symptoms of PE.
Pre-eclampsia is associated with Helicobacter pylori seropositivity in Italy
A more recent study, carried out in 2013 and recently published on Haelicobacter, starting from the epidemiological association between CagA-positive H. pylori strains and pre-eclampsia, hypothesized that anti-CagA antibodies may recognize antigens of cytotrophoblast cells, thus impairing their function. Cytotrophoblast cells were cultured in a medium containing increasing concentration of polyclonal anti-CagA antibodies. Anti-CagA antibodies recognized β-actin of cytotrophoblast cells, showing a dose-dependent binding.

Research shown that some antibodies produced as a result of an infection supported by more aggressive strains of Helicobacter pylori may damage the cells that give rise to the placenta, which, as a consequence, would not grow normally anymore. This would therefore trigger a reduced placental development that could lead to pre-eclampsia. Francesco Franceschi, Professor of the Institute of Internal Medicine and Geriatrics, Catholic University of Rome and first author of the study , affirmed that their study wanted to verify if at the basis of that epidemiological association there was a cross-reaction between anti-CagA and antigens of the trophoblast. In practice, the anti-CagA could recognize a protein of the trophoblast similar to the one expressed by the bacterium, altering its function. This cross-reaction has been clearly demonstrated and the protein which was found to be responsible for is the beta-actin, which is expressed in trophoblast not only in the cytoskeleton but also on the cell surface. It is interesting to observe how the link between anti-CagA and the beta-actin of the trophoblast has been able to significantly reduce its invasiveness in the uterine wall. In addition, the results of recent research carried out at the laboratories of Gynecology showed an antibody damage (anti-CagA) also in the process of endometrial angiogenesis, that together with the invasion of the trophoblast, regulates the process of placentation. It is therefore possible to hypotize that the reduced invasiveness of the trophoblast into the uterine cavity and the reduced endometrialangiogenesis, caused by the binding with the anti-CagA might, in women infected by CagA-positive strains of Helicobacter pylori, lead to a defective system of trophoblast and a defective formation of the placenta and may lead to pre-eclampsia.

Antibodies Anti-Caga Cross-React with Trophoblast Cells: A Risk Factor for Pre-Eclampsia?
Conclusion
Preliminary results of these two clinical studies concerning women with pre-eclampsia found an association between positivity for Helicobacter pylori infection and alteration of the process of placentation. This information could allow early identification of women at risk of developing a pre-eclamptic pregnancy with optimization of its diagnosis and therapy.