GISELLA MARENGO
BEATRICE PUGLIESE
Xanthines belong to the chemical class of purine bases that include some very important endogenous substances such as guanine, adenine, hypoxanthine and uric acid. The noun "xanthines" comes from the greek “xantos”, which means yellow, due to the color that take these compounds when they are dried in the presence of nitric acid.
Xanthines most important from a medical point of view are: aminophylline, theophylline, theobromine and caffeine, that are methylated xanthines and are often called methylxanthines. It is chemically similar alkaloids widely distributed in the plant world.
Theophylline and caffeine are found in the leaves of "thea sinensis", a shrub native to southern China from which we get tea; Theobromine is contained in the seeds of "Theobroma cacao" with which you get the cocoa and chocolate. Caffeine is found in the fruits of "Coffea arabica" and similar species from which it derives the coffee.

In recent years, there is renewed interest in natural methylxanthines and their synthetic derivatives, particularly after the improvement of knowledge on the cellular basis of their actions.
Syntheses

Xanthine (3,7-dihydro-purine-2,6-dione), is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine
Xanthine is a product on the pathway of purine degradation.
It is created both from guanine by guanine deaminase that from adenosine by adenosine deaminase. Purine nucleotides are degraded by a sequence of reactions in which the phosphate group is lost by the action of 5'-nucleotidase. The adenylate is converted into adenosine, which is then deaminated to inosine by adenosine deaminase. The inosine is hydrolyzed in its purine base hypoxanthine and D-ribose. The free hypoxanthine is subsequently oxidized to xanthine and then to uric acid by xanthine oxidase, a flavoenzima that contains in his prosthetic group one atom of molybdenum and iron-sulfur centers . The catabolism of GMP generates uric acid as a final product. The GMP is first hydrolyzed to form the nucleoside guanosine, which is then cleaved releasing the guanine. The free base is removed by hydrolysis of the amino group, generating xanthine that is converted to uric acid by xanthine oxidase.
(David L. Nelson - Michael M. Cox, I principi di biochimica di Lehninger, V edizione).
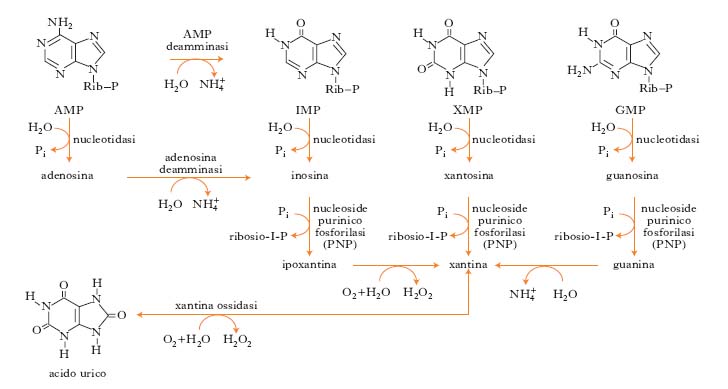
By xanthine derive methylxanthines (theobromine, caffeine and theophylline), whose structure is characterized by a methyl-substituted purine base, or by a heterocycle compound obtained from condensation between the pyridine and the imidazole. The number and position of the methyl substituents differs methylxanthines: caffeine is 1,3,7-trimethyl xanthine; theophylline is 1,3-dimethyl xanthine; theobromine is a 3,7-dimethyl xanthine.
Action & molecular mechanism
The main mechanism of action of xanthine is represented by the inhibition of phosphodiesterase, enzyme that breaks a phosphodiester bond. The pharmacological activity of xanthine is expressed in smooth muscle, heart muscle, central nervous system and kidney. The action on bronchial smooth muscle is relevant in the treatment of asthma.
What is Asthma?
Asthma is a disease characterized by nonspecific hyperactivity of tracheobronchial tree triggered by stimuli of various kinds (allergens, acetylsalicylic acid, smog and respiratory infection). The cause of hyperresponsiveness is unknown, but it is believed that inflammation plays a key role. The airway obstruction is episodic and reversible; the intensity varies from mild and without limitation to the activity of the patient to severe, putting in danger the life of the patient. A severe obstruction persisting for days or weeks is called status asthmaticus. (HARRISON, Principi di medicina interna Il Manuale, XVI edizione).
p=. 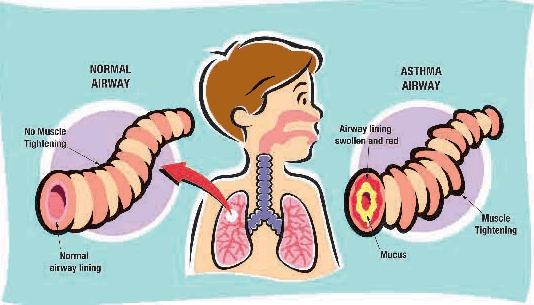
Methylxanthines in asthma therapy
The main methylxanthines used in the treatment of asthma are the Theophylline and Aminophylline.
THEOPHILLINE
https://www.google.it/search?q=theophylline+drug&espv=2&biw=1242&bih=606&source=lnms&tbm=isch&sa=X&ei=M-Y_VOq0Bcz7av_vgbAN&ved=0CAYQ_AUoAQ#facrc=_&i
Theophylline was first extracted from tea leaves and chemically identified around 1888 by the German biologist Albrecht Kossel. It is naturally found in cocoa beans; trace amounts of theophylline are also found in brewed tea.
Theophylline has a diuretic effect and relaxing smooth muscle, particularly at the level of the airway. The diuretic action of theophylline is used in herbal teas and dietary products draining. In the pharmaceutical field, the derivatives of theophylline are used predominantly in the treatment of bronchial asthma, as well as in the treatment of COPD and sleep apnea in children.
The main actions of theophylline :
- relaxing bronchial smooth
- increasing heart muscle contractility and efficiency (positive inotropic)
- increasing heart rate: (positive chronotropic)1
- increasing blood pressure
- increasing renal blood flow
- anti-inflammatory effects
- central nervous system stimulatory effect mainly on the medullary respiratory center.
Mechanisms of action
Theophylline, like other methylxanthines, inhibits in a non-selective phosphodiesterase: this leads to increased intracellular cAMP, activation of PKA, inhibition of TNF-alpha and leukotrienes. It consequently reduces inflammation.
Theophylline is also a potent antagonist of adenosine at the receptors (A1, A2, A3): this activity reduces the bronchoconstriction typical of asthmatic patients.
Pharmacokinetic
When theophylline is administered intravenously, its bioavailability is almost maximum and is distributed in the extracellular fluid, placenta, into breast milk and the central nervous system.
The metabolism of this methylxanthine occurs in the liver where it undergoes a demethylation by cytochrome P450.
Theophylline is excreted in the urine. The clearance of the drug is increased in children, adolescents, smokers, cystic fibrosis and hyperthyroidism, while decreases in the elderly, in acute congestive heart failure, cirrhosis, hypothyroidism and febrile viral disease.
The adverse effects
When it is combined with other drugs, the use of theophylline may lead to complications such as nausea, diarrhea, increased heart rate, arrhythmias, and excitement of the central nervous system (headache, insomnia, irritability, dizziness). Its use should therefore be monitored by direct measurement of its serum levels to avoid toxicity.
The best known and most widely used derivatives of theophylline is Aminophylline
http://en.wikipedia.org/wiki/Theophylline
AMINOPHILLINE
As demonstrated in a recent study, (Aminophilline suppress the release of chemical mediators in treatment of acute asthma., 2006) aminophylline, a theophylline derivative, could act as an anti-inflammatory agent as well as a bronchodilator in the treatment for acute asthma exacerbations.

Mechanism of Action & Pharmacodynamic
This drug is obtained from the association chemistry between theophylline and ethylenediamine, characterized by the large solubility in an aqueous environment in contrast to the pure theophylline, whose chemical nature alkaloid, will limit greatly the hydrophilicity, thus making it less suitable for therapeutic purposes.
After oral or parenteral administration, the Aminophylline performs a therapeutic action comparable to that of Theophylline, resulting in inactivation of the enzyme phosphodiesterase, with a consequent increase in the concentrations of intracellular cyclic AMP and subsequent bronchodilation. This increase is directly related spasmolytic activity on the smooth muscle bronchial.
The above-mentioned activities, combined with the antihistamine activity, effective in reducing edema associated with the asthmatic condition and hypersecretion of mucus, causes a marked improvement in the airway and it restores the normal ventilatory capacity.
Derivatives metilxantines after hepatic metabolism, are eliminated in the urine.
The improvement of respiratory function depends also from the intense stimulation of the bulbar respiratory centre, which results in an increase in the rhythm and amplitude of respiration. The aminophylline reduces also systemic blood pressure and pulmonary and exerts positive chronotropic and inotropic effects, which result in an increase in cardiac output.
Instructions for use and dosage
The Aminophylline is located in vials for intravenous use 240 mg per 10 ml, in vials for intramuscular use from 350 mg to 2 ml of solution or in suppositories of 350 mg Aminophylline. The therapy is reserved for patients with asthma or respiratory problems with bronchospastic component.
Dosages depend on the mode of administration and the physio-pathological characteristics of the patient. In adults, the treatment is applied to cases of serious bronchial asthma and is achieved through the slow infusion of a solution obtained by diluting 480 mg of aminophylline (equal to 2 vials of 240 mg / 10 ml) in 50 ml of a solution for infusion. The total dose given will not exceed 0.8 ml / kg (equal to 5.6 mg aminophylline / kg). In any case, the intravenous administration of the medicine should be performed with the patient in supine and with controlled slow (15-20 minutes). In children, however the dosage is lower (1 mg / kg / hour).
Warnings

Aminophylline therapy should be carefully monitored in order to identify any potential side effects or contraindications to the use of this drug. Particular caution should be given to patients with heart disease, hypertensive, hypoxemic or hyperthyroid, or suffering from impaired liver and kidney function.
Aminophylline should not be administered concurrently with other xanthine compounds; the association between aminophylline and ephedrine or other sympathomimetic bronchodilators requires caution
The anticonvulsant drugs and cigarette smoking may increase the clearance of aminophylline in reducing the plasma half-life. In such cases, it may be necessary to increase the aminophylline dosage.
The coadministration with halothane (increased cardiac toxicity), epinephrine (increases the toxicity level of the central nervous system and gastrointestinal tract), ketamine (lowers the seizure threshold) increases its toxicity. Aminophylline also decreases the effectiveness of benzodiazepines.
Although negative effects of aminophylline on the developing fetus are not known, the use in pregnancy should be limited to cases in which asthma is a serious danger to the mother.
It is not recommended to take the medication during breastfeeding because of the ability of theophylline to concentrate in breast milk.
Side Effects

The administration of Aminophylline may determine the occurrence of transient side effects such as nausea, vomiting, epigastric pain, headache, irritability, tachycardia, insomnia, and tachypnea. Less frequently observed events of cardiovascular origin (atrial fibrillation, syncope, etc.), psychiatric disorders (irritability, depression, anxiety and agitation) and alterations in renal and urinary system. Sometimes the side effects are attributable to an overdose of the drug. (Manuale Merck di diagnosi e terapia, VI edizione;)