DESCRIPTION
Obeticholic acid (OCA), is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid (6E-CDCA). It has also been known as INT-747. It is undergoing development as a pharmaceutical agent for several liver diseases and related disorders such as primary biliary cirrhosis (PBC), non alcoholic steatohepatitis (NASH), portal hypertension and bile acid diarrhea.

CLASSIFICATION
NAME: Obeticholic Acid
TYPE/GROUP: Obeticholic acid is a semi-synthetic analogue of the primary bile acid chenodeoxycholic acid (CDCA)
CATEGORY:
- anti-cholestatic
- anti-inflammatory
- anti-fibrotic
MOLECULAR FORMULA: C 26 H 44 O 4
MOLECULAR WEIGHT: 420.62 g/mol
IUPAC NAME: (3α,5β,6α,7α)-6-Ethyl-3,7-dihydroxycholan-24-oic acid
MOLECULAR MECHANISM OF ACTION:
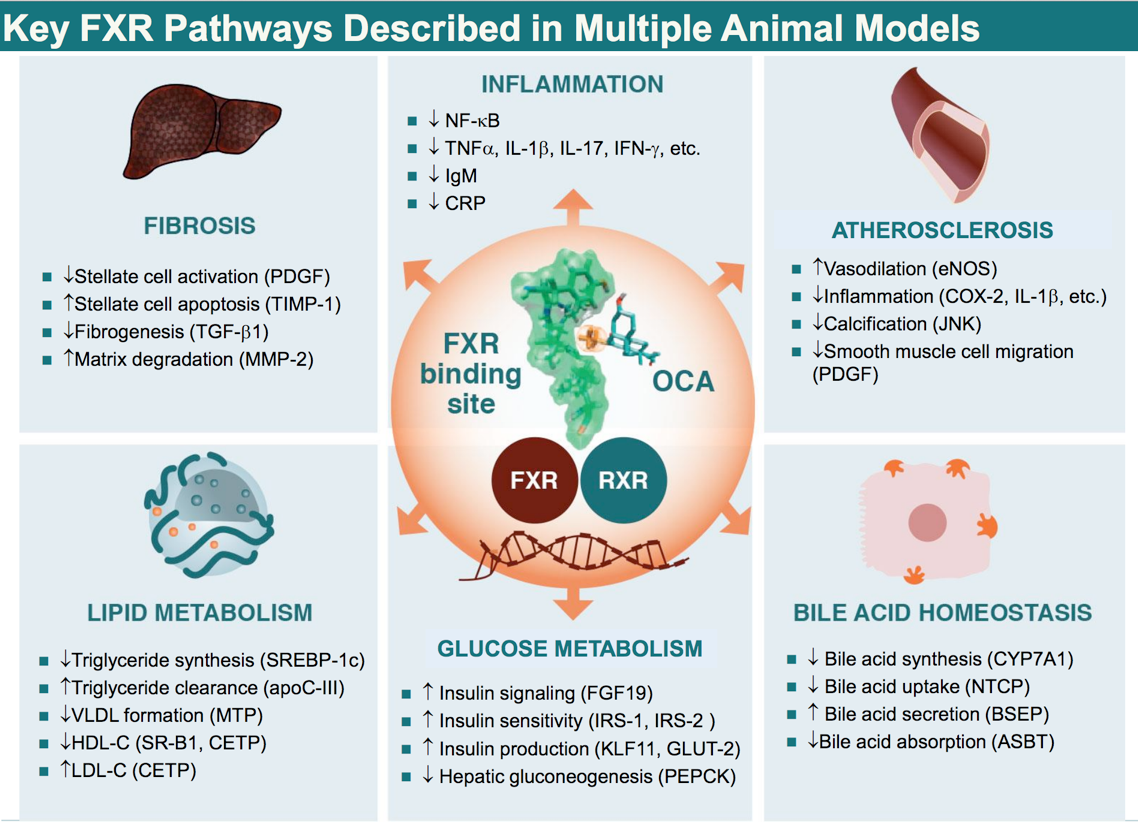
Obeticholic acid is a novel derivative of chenodeoxycholic acid, the primary human biliar acid (BA). OCA is an FXR agonist, a nuclear receptor predominantly expressed in the gastrointestinal tract (liver and intestine) that has an important role in the enterohepatic circulation of BAs. The FXR is the key intracellular BA sensor regulating several metabolic processes involved in BA formation, transport and detoxification.
FXR exerts its functions by eliciting transcriptional alteration. The protein has a multidomain structure with distinct regions engaged in DNA binding, ligand binding and transactivation.
Ligand bindings results in dissociation of co-bound co-repressor and recrutiment of co-activator proteins, thus, promoting target gene expression. (video of the mechanism)
(December 2014; Obeticholic acid for the treatment of primary biliary cirrhosis)
INTESTINE:
Some of the effects of FXR (previously activated by FXR-agonist such as OCA) are mediated by the enterokine FGF19. FGF-19 is an unusual member of the FGF family in being secreted into the portal circulation and having an endocrine mode of action.
After portal delivery, FGF-19 signals in the liver via hepatocytic FGF receptor 4 (FGFR4); FGFR4 is a tyrosine kinases receptor that exerts its effects on target gene expression via activation of signalling cascades including the mitogen-activated protein kinase pathways.
Ileal bile acid uptake induces FGF-19 expression via FXR-mediated transcrptional induction.
FGF-19 regulates bile acid synthesis via downregolation of CYP7A1 expression in a SHP-dependent way.
LIVER:
In the liver, FXR negatively regulates bile acid production by repressing CYP7A1, the rate-limiting enzyme of the synthetic pathway of bile acids, and inhibiting HMG-CoA reductase and lanosterol 14 α-demethylase , which play key roles in the synthesis of cholesterol. FXR induces the expression of BSEP and MRP2, which are involved in bile acid export, and simultaneously represses bile acid import by downregulating NTCP and OATP2/8. In the intestine, FXR induces the expression of IBABP, which provides the enterocytes with a shuttling device for the delivery of bile acids from the apical to the basolateral membrane. FXR does not directly regulate the ASBT gene, but it may influence the network of transcription factors that are involved in a response to bile acids. FXR activates the expression of OST α / ß, which serves to transport bile acids from the gut to the enterohepatic circulation where they are transported back to the liver.
(27 August 2013 - Nature Reviews Gastroenterology & Hepatology; Bile acid receptors as targets for drug development)

OCA is approximately 100 times more potent as an FXR ligand than chenodeoxycholic acid (CDCA). Ursodeoxycholic acid (UDCA) is the only approved drug to treat PBC. UDCA works by breaking up more detergent bile acids into pool. By lowering the increase in bile acids, UDCA is able to effectively alleviate the symptoms associated with PBC like itching.
In contrast to UDCA, OCA reduces the elevated bile acid by a different mechanism. In specific, OCA activates FXR with 100x potency compared to natural bile acid, namely CDCA. OCA is not only able to displace the detergent bile acids pool, but it also suppresses the inflammation that causes the destruction of the bile ducts in the liver.

.
The enhanced potency of OCA results from the filling of a hydrophobic pocket by the 6α-ethyl moiety respect to the chenodeoxycholic acid (CDCA).
(April 2013 - interceptpharma; The Development of Drugs Interacting with Bile Acid Receptors.)

Activation of FXR inhibits BA synthesis from cholesterol and also protects against the toxic accumulation of BAs through increased conjugation in the liver and secretion into the bile canaliculi. These mechanisms limit the overall size of the circulating bile pool while promoting choleresis, thus reducing hepatic exposure to BAs.
(29 May 2012; Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis)
STUDY, ANALYSIS AND RESULTS
ANIMAL DISEASE MODELS:
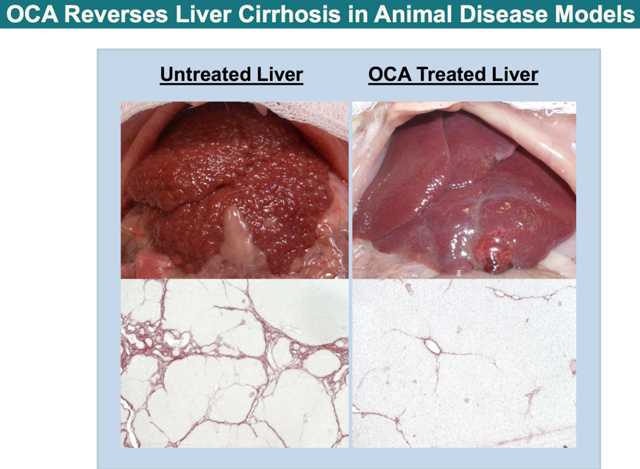
Sprague-Dawley rats received thioacetamide (TAA) for 4 weeks to induce fibrosis, then started OCA (5mg/kg) added to ongoing TAA for 4 weeks vs control. Results show that OCA reverses liver cirrhosis. Moreover OCA improves triglycerides, steatosis and inflammation in animal models of metabolic syndrome and NAFLD: OCA treatment reduced TG levels at week 12 (231 mg/ml) vs. no treatment (305 mg/ml).
Friedman et al., AASLD Presentation, 2005
(September 2014 - Interceptpharma; Building a Specialty Focused Business in Chronic Liver and Intestinal Diseases with High Unmet Medical Need.)
CLINICAL TRIAL:
Obeticholic acid in PBC Preliminary studies of OCA in patients with PBC have been encouraging thus far. The first Phase II, multicenter, double-blind randomized controlled study was conducted in 165 patients with PBC who were non-responders to UDCA, characterized by ALP above 1.5 times upper limits of normal on appropriate therapy. Three different doses of the drug, 10, 25 or 50 mg, or placebo, were given in addition to UDCA, for a total of 12 weeks. Patients who received OCA experienced substantial reduction in ALP compared to the placebo group (21 - 25 vs 3% reduction). Furthermore, gamma-glutamyl transpeptidase (GGT), aminotransferase and IgM levels were significantly decreased compared to those who received placebo. A second small Phase II study included 59 UDCA-naïve patients who were randomized to placebo or OCA at 10 or 50 mg/day for 12 weeks. Patients who received OCA experienced a substantial reduction in ALP compared to placebo (45% vs no change). As in the first study, aminotransferases, GGT and IgM levels were significantly decreased with OCA. A Phase III, multicenter study of OCA in patients with PBC who are non-responders to UDCA or intolerant to UDCA is currently underway (clinicaltrials.gov, NCT01473524). (2 Jan 2014; Obeticholic acid and budesonide for the treatment of primary biliary cirrhosis)

SIDE EFFECTS:
Pruritus was the most common adverse event, occurring in 50% of patients receiving placebo and a similar percentage of patients at 10 mg; however, > 80% of patients receiving the two higher doses complained of pruritus, leading to discontinuation of the drug in up to 24% of cases.
CONCLUSION
In conclusion, the randomized controlled clinical trial data demonstrating
biochemical efficacy of OCA, a FXR agonist, when given to patients with PBC with an
inadequate response to UDCA therapy. Across all doses tested, biochemical efficacy of
OCA was evident; based on the balance of efficacy and tolerability, in this study 10 mg
once daily of OCA was the most effective dose, and has thus formed the basis for further
studies of OCA in PBC. Evaluating the lower end of the dose response relationship in
treatment of PBC is merited, as is a strategy of titrating the dose of OCA based on an evaluation that includes biochemical markers and symptomatic response to low doses of the
drug. The trial therefore supports ongoing efforts to further evaluate the long term safety
and clinical efficacy of OCA as a new therapy for patients with PBC.
(December 2014; Efficacy of Obeticholic Acid in Patients With Primary Biliary Cirrhosis and Inadequate Response to Ursodeoxycholic Acid)