Francesca Brignardello
Alessandro Godono
DESCRIPTION
Arginine vasopressin receptor antagonists (Vaptans) are a class of drugs that interfere with action at the vasopressin receptor. They are used in the management of hyponatremia, especially in patients with congestive heart failure, liver cirrhosis and SIADH.
Introduction
Hyponatremia, defined as an excess of water in relation to sodium in the extracellular fluid, is the most common electrolyte disorder in hospitalized patients, affecting one in five of all of them. It is defined as serum sodium concentration <135 mEq/L.
About 85–90% of all sodium is extracellular; therefore, extracellular fluid volume (ECF) is a reflection of the total sodium content in the body. Hyponatremia usually reflects retention of excessive water relative to sodium rather than sodium deficiency alone.
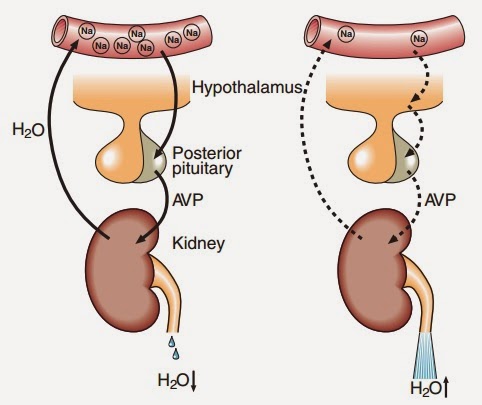
Mechanisms of osmoregulation and volume regulation help to maintain water balance and tonicity in the body. Interplay of the renal action of arginine vasopressin (AVP) or antidiuretic hormone (ADH), renin angiotensin aldosterone system (RAAS), sympathetic nervous system (SNS), and thirst help to control water homeostasis in the body and keep plasma osmolality between 275 and 290 mOsm/kg water.
Vaptans: A new option in the management of hyponatremia, 2012
Hyponatremia can be associated with low, normal, or high tonicity:
- Isotonic hyponatremia refers to the expansion of ECF with isotonic fluids that do not contain sodium (e.g., mannitol).
- Hypertonic hyponatremia or pseudohyponatremia occurs when there is increase in effective osmoles in ECF with shift of water from the cells to ECF (e.g., hyperglycemia in insulin-resistant states, dyslipidemia or paraproteinemia) called translocational shift. Hypotonic hyponatremia is the most common form of hyponatremia and occurs either due to impaired renal excretion of water or excess intake of water in the presence of normal renal excretion.
- Hypotonic hyponatremia is of three types depending on the ECF volume associated with it.
- Hypovolemic hyponatremia is characterized by low serum sodium associated with contraction of plasma volume, signs of dehydration such as tachycardia, orthostatic hypotension and flat neck veins.
- In euvolemic hyponatremia, there is relative gain of water due to impaired water excretion combined with increased intake. Physical examination reveals absence of features of ECF overload (e.g. edema, ascites, pulmonary edema) or ECF volume depletion (tachycardia, low blood pressure, flat neck veins). Syndrome of inappropriate antidiuretic hormone secretion (SIADH) is the most common cause of euvolemic hyponatraemia and is often poorly understood and inappropriately treated. It is usually a result of medication, neurological, respiratory or malignant disease.
- Hypervolemic hyponatremia is due to expanded ECF volume and plasma with ascites and edema. There is disproportionate renal retention of water with respect to the retention of sodium.
Depending on disease stage and severity, the electrolyte disorder can present with a wide spectrum of neurological signs and symptoms, ranging from adynamia and gait disturbances, to syncope or coma.
The introduction of vasopressin antagonists, or vaptans, into clinical practice heralds the beginning of a new and exciting era for this important group of disorders.
SIADH and hyponatraemia: foreword, 2009
Use of Vaptans in the Management of Hyponatremia
Combined with conventional therapy, vasopressin receptor antagonists (AVP-R antagonists) are able to increase the excretion of electrolyte-free water and the sodium concentration.
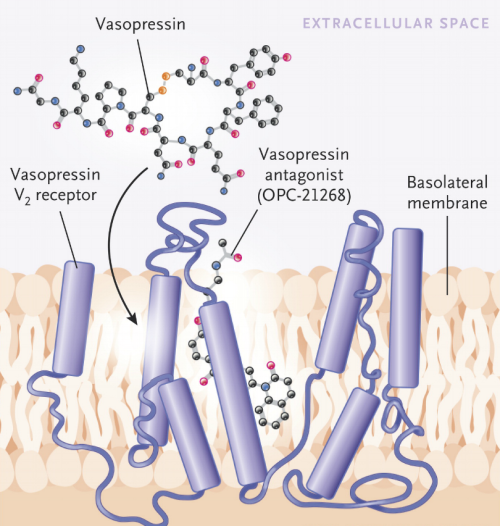
Arginine-vasopressin (AVP) is a hormone that plays an important part in circulatory and water homoeostasis. The three arginine-vasopressin-receptor subtypes—V1aR, V1bR, and V2R—all belong to the large rhodopsin-like G-protein-coupled receptor family.
The three subtypes of AVP receptors differ in localization and signal transduction mechanisms:
- The V1aR is a Gq-coupled receptor that activates phospholipase C and increases cytosolic free calcium. It is expressed in vascular smooth muscle cells and causes vasoconstriction; furthermore, constriction in renal mesangial cells reduces glomerular flow and increases tubular sodium reabsorption.
- V1bR was found in the anterior pituitary gland and is involved in ACTH release.
- The V2R has a key role in the kidney, but there are numerous extrarenal V2R whose clinical importance spans widely from the stimulation of coagulation factors in the endothelium to V2R-regulated membranous fluid turnover in the inner ear, V2R-regulated mitogenesis and apoptosis in certain tumour tissues, and numerous other cell types where the physiological role of V2Rs still requires further research. In the principal cells of the renal collecting and connecting tubules, V2R mediate the hydro-osmotic effect of vasopressin. The binding of AVP to the renal V2R activates the Gs adenylyl cyclase system, increasing intracellular levels of cyclic 3′, 5′- adenosine monophosphate. The latter stimulates protein kinase A that phosphorylates preformed aquaporin-2 (AQP2); water channels localized in intracellular vesicles. Phosphorylation promotes their translocation to the apical membrane and the final effect is increased water reabsorption.
History
The development of desmopressin, the longer acting AVP V2 agonist, focused attention on synthesizing vasopressin receptor antagonists, which showed either vasodilatory or diuretic properties. The first peptide vasopressin receptor antagonists were reported by Manning et al in 1981 and initially studied in rats.
Synthetic antagonists of in vivo antidiuretic and vasopressor responses to arginine-vasopressin, 1981
The investigation of peptide vasopressin receptor antagonists in several species confirmed the observation of aquaresis, which is the term applied to the free-water clearance with little or no change in urinary solute excretion. However, a paradoxical agonist effect on long-term administration resulting in water reabsorption restricted their clinical use in humans.
Yamamura et al made an important contribution in the search for a clinically useful aquaretic in 1992 when they reported the first nonpeptide V2 receptor antagonist, OPC-31260. This compound avoided the clinical shortcomings of its peptide predecessors due to its longer half-life and good oral bioavailability and because it had no agonist effect. The highly selective OPC-31260 blocked AVP-induced cAMP production. The compound had a potent dose-dependent aquaretic effect in normally hydrated men.
Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist, 1992
It resulted in a safe and effective increase in serum sodium level in patients with hyponatremia due to SIADH. Several other vaptans have been developed and tested in large clinical trials.
Mechanism of action
The binding of arginine vasopressin to the vasopressin V2 receptor (V2R) stimulates a Gs-coupled protein that activates adenylyl cyclase, in turn causing production of cyclic adenosine monophosphate to activate protein kinase A. This pathway increases the exocytosis of vesicles containing aquaporin water channels and inhibits endocytosis of the vesicles, both of which result in increases in aquaporin-2 channel formation and apical membrane insertion. This allows an increase in the permeability of water from the collecting duct. Vasopressin V2 receptors block this effect, and thus the collecting duct remains impermeable to water and free water excretion increases.
The nonpeptide vasopressin receptor antagonists, or vaptans, block the effect of AVP on the collecting ducts and cause water diuresis.
The stable expression of vasopressin receptors in cell lines has allowed for the study of their molecular mechanisms. Thibonnier et al. developed a three-dimensional model of the receptor and docked vasopressin in its binding site.
A molecular model of agonist and nonpeptide antagonist binding to the human V(1) vascular vasopressin receptor, 2000.
Using site-directed mutagenesis and affinity binding, the investigators established the site on the receptor at which antagonists are likely to disrupt binding to the V1A and V2 receptors. Vasopressin binds at a superficial site, whereas the antagonist penetrates deeply into the membrane, altering the ability of the agonist to bind to the receptor in the adjacent loop. This inhibition culminates in the prevention of the insertion of water channels into the apical membrane, which thereby inhibits the reabsorption of water and the generation of concentrated urine.
Lixivaptan: a vasopressin receptor antagonist for the treatment of hyponatremia, 2012

FDA-Approved Nonpeptide Vasopressin Antagonists
Tolvaptan
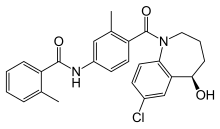
Approved for up to 30 day use for euvolemic and hypervolemic hyponatremia; available as 15- and 30-mg tablets for oral use; starting dose of 15 mg by mouth daily, may be increased by 15 mg each day up to a maximum of 60 mg/d based on effect on serum sodium level; therapy should be initiated and reinitiated in a hospital setting and fluid restriction omitted on the first day. Tolvaptan was approved by the U.S. Food and Drug Administration (FDA) on May 19, 2009, and is sold by Otsuka Pharmaceutical Co. under the trade name Samsca.
Sistematic (IUPAC) name: N-(4-{[(5R)-7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]carbonyl}-3-methylphenyl)-2-methylbenzamide
Formula: C26H25CIN2O3
Molecular mass: 448.941 g/mol
ATC code: C03XA01
Trade name: Samsca ®*10 cpr 15 mg/*10 cpr 30 mg
Pharmacokinetic properties
Bioavability: Unknown (40% absorbed)
Protein binding: 99%
Metabolism: Hepatic
Primarily metabolized by CYP3A4 and is a substrate and inhibitor of P-glycoprotein; simultaneous use with other CYP3A4 inhibitors should be avoided; contraindicated with strong CYP3A inhibitors; reduced drug effect if used with inducers of CYP3A4, including phenytoin and rifampin.
Biological half-life: 12 hours (terminal)
Route of administration: oral
Conivaptan
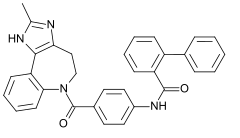
Approved for short-term use in hospitalized patients with euvolemic or hypervolemic hyponatremia; available as 20-mg premixed formulation in 100 mL of 5% dextrose; loading dose, 20-mg IV bolus over 30 minutes, then 20-mg infusion over 24 hours; based on effect on serum sodium, the continuous infusion dose may be increased to a maximum of 40 mg/or omitted; duration of treatment should not exceed 4 days. Conivaptan was approved by the U.S. Food and Drug Administration (FDA) on December 2005 for the treatement of euvolemic hyponatremia and in February 2007 the indication was extended to include hypervolemic hyponatremia.
Sistematic (IUPAC) name: N-(4-((4,5-dihydro-2-methylimidazo[4,5-d]benzazepin- 6(1H)-yl)carbonyl)phenyl)- (1,1'-biphenyl)-2-carboxamide
Formula: C32H26N4O2
Molecular mass: 498.583 g/mol
ATC code: C03XA02
Trade name: Vaprisol ®*20 mg per 4-mL ampule
Pharmacokinetic properties
Bioavability: Unknown
Protein binding: 99%
Metabolism: Hepatic
CYP3A4 was identified as the sole cytochrome P450 isozyme responsible for the metabolism of conivaptan. Four metabolites have been identified.
Biological half-life: 5 hours (terminal)
Route of administration: intravenous
Pharmacodynamic properties
Conivaptan and tolvaptan have differing affinities for the vasopressin receptor.
Conivaptan is twice as potent as tolvaptan as an inhibitor of the V1receptor, but tolvaptan is 2.5 times as potent an inhibitor of the V2receptor. The relative inhibition of the two receptors (V2:V1 selectivity ratio) is much greater with tolvaptan (by a factor of 29) than with conivaptan (by a factor of 5.7). Thus, conivaptan is a nonselective vasopressin inhibitor, whereas tolvaptan is a more selective V2 inhibitor.
Therapeutic indications
They are the only agents that promote excretion of electrolyte-free water by directly blocking binding of AVP to its renal receptors. They currently have a potentially important role in the treatment of dilutional (euvolemic and hypervolaemic) hyponatremia. The persistent secretion of AVP, despite low serum osmolality, in patients with CHF, cirrhosis, and SIADH makes vaptans a therapeutic option for these patients.
Side effects
| Tolvaptan | Conivaptan |
| Constipation | Constipation |
| Diarrhea | Asthenia |
| Dry mouth | Dry mouth |
| Headache | Pyrexia |
| Thirst | Thirst |
| Nausea | Anorexia |
| Trouble sleeping | Hyperglicaemia |
| Vomiting | Pollakiuria or Polyuria |
Severe allergic reactions may manifestate with rash, hives, itching, difficulty breathing, tightness in the chest, swelling of the mouth, face, lips, or tongue
Contraindications
Contraindications for the vaptans include symptomatic hyponatraemia requiring an infusion of hypertonic saline, hypovolaemic hyponatraemia and the use of strong CYP3A4 inhibitors (e.g. ketoconazole). Patients with hypovolaemic hyponatraemia should be treated with volume repletion to restore euvolemia. With restoration of adequate circulatory volume, the focus of management often becomes prevention of over-rapid correction, a process that includes frequent monitoring of serum sodium levels, urine output, and urine osmolality; liberalization of fluid intake; and administration of hypotonic intravenous fluids.
Vaptans should not be relied upon when severe neurologic symptoms such as seizures or a markedly altered sensorium are present. Vaptans should not be used together with hypertonic saline solution, and the initiation of a vaptan should be delayed until the response to hypertonic saline solution is complete and serum sodium level has reached a plateau. This approach reduces the chance of uncontrolled over-rapid correction during the patient's early course when serum sodium level is lowest and the risk of osmotic demyelination is highest. Particular vigilance is advisable in cases in which SIADH occurs in the setting of pituitary surgery or injury and the possibility exists that diabetes insipidus might subsequently arise.
Conclusions
Vaptans in many ways are superior to conventional treatments for hyponatremia, which is a common clinical problem, and traditional treatments are often ineffective. However, many unresolved questions remain. Vaptans have not been shown to affect mortality or alter the disease processes of CHF or cirrhosis, in which hyponatremia frequently develops. Their high price is a major barrier to routine use. Although these agents show much promise, further study is needed to clarify whether vaptans can improve the morbidity and cost burden of hyponatremia. Limitations aside, vaptans are highly effective and well tolerated for the treatment of euvolemic and hypervolemic hyponatremia. Compared with conventional treatments, they more elegantly and precisely target the disordered physiology responsible for hyponatremia. Clinicians should familiarize themselves with this class of drugs, because prescribing of vaptans is likely to increase in the future.
Role of Vaptans in the Management of Hyponatremia, 2013