DEFINITION
A short protein description with the molecular wheight, isoforms, etc...
Use, when available, the link to Wikipedia (Es Trypsin)
External links not available on Wikipedia have to be added here
THE GENE
Wikigenes includes links to
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage
The Protein Aminoacids Percentage gives useful information on the local environment and the metabolic status of the cell (starvation, lack of essential AA, hypoxia)
Protein Aminoacids Percentage
PPA

PPA extracellularly cleaved

SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,

The APP copper binding

A) APP indicating the CuBD sequence, Cu binding coordination of histidine, and cysteine in red.
B) Left: Cu uptake by Cu transport proteins. Center: Cu uptake in conditions of high extends copper. Copper interacts with cell membranes causing an increased in oxidative damage. Right: Cu uptake in the presence of the CuBD of APP decreased oxidative stress generated by copper. (The N-terminal copper-binding domain of the amyloid precursor protein protects against Cu2+ neurotoxicity in vivo, 2006)
Pleiotropic effects of beta-amyloid
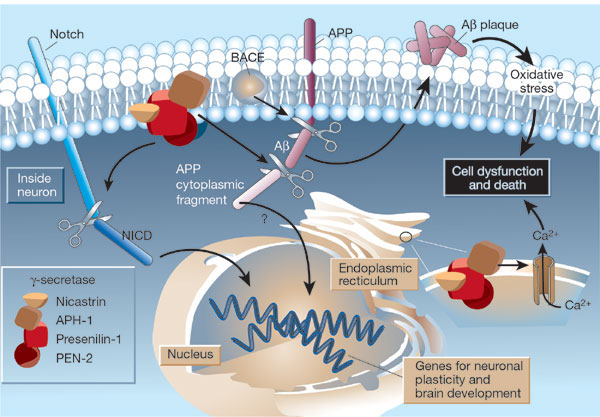
The g-secretase complex is formed by:
- Presenilin-1
- nicastrin
- APH-1
- PEN-2
It is located in the plasma membrane and endoplasmic reticulum (ER) of neurons.
Substrates are:
- Notch that generates a fragment (NICD)
- APP that generates an APP cytoplasmic fragment
that move to the nucleus
Amyloid as Copper source
Intracellular copper deficiency increases amyloid-beta secretion by diverse mechanisms. 2008
AICD and transcriptional effects
 Papers APP and transcriptional
Papers APP and transcriptional
The gamma/epsilon-secretase-derived APP intracellular domain fragments regulate p53. 2007
Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. 2008
Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM., Biochem Soc Trans. 2008 Dec;36(Pt 6):1282-7.
The essential metals iron, zinc and copper deposit near the Abeta (amyloid beta-peptide) plaques in the brain cortex of AD (Alzheimer's disease) patients. Plaque-associated iron and zinc are in neurotoxic excess at 1 mM concentrations. APP (amyloid precursor protein) is a single transmembrane metalloprotein cleaved to generate the 40-42-amino-acid Abetas, which exhibit metal-catalysed neurotoxicity. In health, ubiquitous APP is cleaved in a non-amyloidogenic pathway within its Abeta domain to release the neuroprotective APP ectodomain, APP. To adapt and counteract metal-catalysed oxidative stress, as during reperfusion from stroke, iron and cytokines induce the translation of both APP and ferritin (an iron storage protein) by similar mechanisms. We reported that APP was regulated at the translational level by active IL (interleukin)-1 (IL-1-responsive acute box) and IRE (iron-responsive element) RNA stem-loops in the 5' untranslated region of APP mRNA. The APP IRE is homologous with the canonical IRE RNA stem-loop that binds the iron regulatory proteins (IRP1 and IRP2) to control intracellular iron homoeostasis by modulating ferritin mRNA translation and transferrin receptor mRNA stability. The APP IRE interacts with IRP1 (cytoplasmic cis-aconitase), whereas the canonical H-ferritin IRE RNA stem-loop binds to IRP2 in neural cell lines, and in human brain cortex tissue and in human blood lysates. The same constellation of RNA-binding proteins [IRP1/IRP2/poly© binding protein] control ferritin and APP translation with implications for the biology of metals in AD. Local Fulltext
biological function
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC USE
APP
Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease 2007 B de Strooper


The recent discovery by Cao and Südhof linking the catabolic product of APP cleavage to transcription activation may illuminate the processing of APP as a model of RIP signaling. (A Transcriptively Active Complex of APP with Fe65 and Histone Acetyltransferase Tip60 2001) Currently, two substrates, APP and Notch, have been identified in association with PS-1/{gamma}-secretase cleavage; two other substrates, SREBP and ATF6, are associated with RIP processing by other proteases.
Co-localization of the amyloid precursor protein and Notch intracellular domains in nuclear transcription factories.