Liraglutide marketed under the brand name Victoza, is a long-acting glucagon-like peptide-1 agonist (GLP-1 agonist an incretin) developed by Novo Nordisk for the treatment of type 2 diabetes. The product was approved by the European Medicines Agency (EMA) on July 3, 2009, and by the U.S. Food and Drug Administration (FDA) on January 25, 2010.
Liraglutide

GENERALITY
Liraglutide improves control of blood glucose.
It reduces meal-related hyperglycemia (for 12 hours after administration) by increasing insulin secretion, delaying gastric emptying, and suppressing prandial glucagon secretion.
Liraglutide is an acylated human glucagon-like peptide-1 (GLP-1) agonist. GLP-1 is secreted by L-cells in small intestine and duodenum. The secretion of this hormone is stimulated by gastrointestinal passage and digestion process. GLP-1 gets to bloodstream and affects a series of organs via its receptor. From the glycemy regulation point of view, one of the most important elements is the influence on the receptor in beta pancreatic cells. Binding to the receptor stimulates insulin secretion, GLP-1 is responsible for the secretion of 50 to 70% of insulin in response to oral glucose administration. . Despite the fact that in response to gastrointestinal passage stimulation, quite big amount of GLP-1 hormone is secreted, only a small part of it reach pancreatic islets. This happens because of action of an enzyme dipeptidyl peptidase-4 (DPP-4) which decomposes GLP-1 in blood within 2–4 minutes.
The strategies of use of incretin hormones in type 2 diabetes treatment include the use of DPP-4 inhibitors and exogenous GLP-1 substitution.
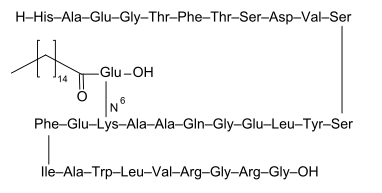
Liraglutide is an analogue of human GLP-1, in 97% homological with a native molecule. The differences between native GLP-1 and liraglutide include exchange of lysine to arginin in 34 position and addition of glutamate to lysine in position 26; to glutamate also was added 16C fatty acid-palmitic acid. After subcutaneous administration, liraglutide forms heptameres which slow down the release of the substance into the bloodstream. In blood, liraglutide is reversibly connected to albumins by the fatty acids. That is why liraglutide has 24 hour long action. It should be also stressed that introduced modifications protect liraglutide from the action of the DPP-4. So the pharmacokinetic profile of liraglutide, which makes it suitable for once daily administration, is a result of self-association that delays absorption, plasma protein binding and stability against metabolic degradation by DPP-IV. The medication is administered once daily in the doses of 0.6 mg (for at least a week), after a week, the patient should augment the doses to 1.2 mg, which is therapeutic dose, however, it is also possible to take the doses of 1.8 mg.
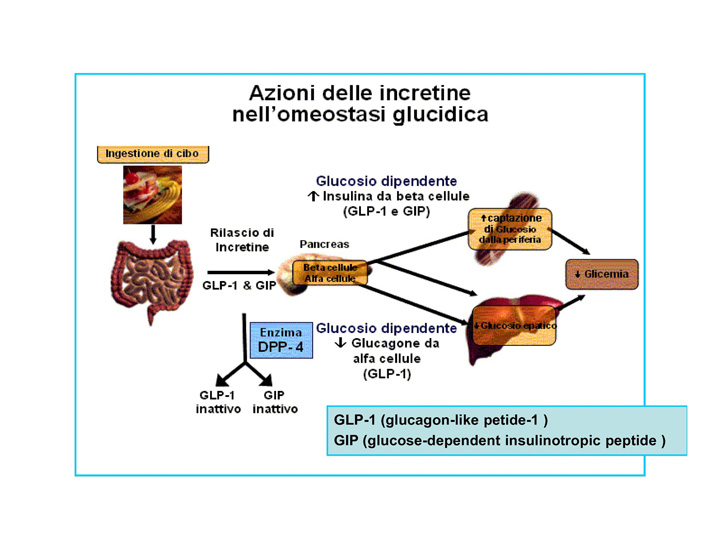
Liraglutide activates the GLP-1 receptor coupled to adenylyl cyclase by the stimulatory G-protein, Gs, in pancreatic beta cells. Liraglutide increases intracellular cyclic AMP (cAMP), leading to insulin release in the presence of elevated glucose concentrations. This insulin secretion subsides as blood glucose concentrations decrease and approach euglycemia. Liraglutide also decreases glucagon secretion.
Liraglutide may have advantages over current therapies:
• It stimulates insulin secretion only when blood glucose levels are higher than normal.
• It has the potential for inhibiting apoptosis and stimulating regeneration of beta cells (seen in animal studies).
• It decreases appetite and maintains body weight
• It lowers blood triglyceride levels.
• It has only mild and transient side effects, mainly gastrointestinal.
Liraglutide
Long term effectiveness and safety of liraglutide in clinical practice,2013
The influence of incretin mimetics on cardiovascular risk factors in diabetes,2012

ACTION ON CARDIOVASCULAR SYSTEM
Type 2 diabetes is related with the risk of cardiovascular disease development which is the major cause of death. Liraglutide not only give the possibility of glycemic control but also could influence the cardiovascular parameters.
The III phase of clinical study of liraglutide has common acronym LEAD (Liraglutide Effect and Action in Diabetes) included 4456 people. The LEAD program covered six randomized studies analyzing the influence of drug administration at different therapeutical stages of type 2 diabetes treatment. The summary of the program is presented in Table1 .As it can be noticed, the liraglutide therapy improved glycemic control reducing the level of glycated hemoglobin by an average 1,13%; the reduction depended both on fasting glucose reduction and postprandial glucose reduction. During the therapy with this medication, very low risk of hypoglycemia was proved which is related to the mechanism of action of liraglutide, it reduces glycemy only on the condition of elevated blood glucose level. The effect of better glycemic control was accompanied by the reduction in systolic blood pressure at an average by 3,41 mmHg, which can be considered as a protective action from further diabetic complications such as cardiovascular complications.
Beneficial effect of incretin medications on cardiovascular system results both from direct and indirect influence on heart and arterial system. The indirect effect is the influence on glycemic control with the lack of the risk of hypoglycemia, arterial blood pressure normalization, and body weight loss. Liraglutide therapy lasting 26 weeks in patients suffering from type 2 diabetes led to significant cholesterol concentration reduction, including LDL fraction, triglycerides, and free fatty acids.
Body weight loss
The summary of the LEAD program showed that liraglutide therapy cause body weight decreases. The mechanisms responsible for body weight loss includes the influence on digestive system and central nervous system. In fact Liraglutide causes reduction of the gastrointestinal passage and stomach emptying and it influences hunger/satiety centers in central nervous system. Both mechanisms, prolonged stomach emptying and satiety centre stimulation in brain, result in the reduction of food intake and body weight loss.
Cholesterol concentration reduction
In diabetic patients is an obvious association between lipid abnormalities and cardiovascular events. Increased levels of triglycerides, LDL and lipoprotein particles are predictors of increased cardiovascular risk.
Liraglutide decreases postprandial triglyceride, apolipoproteins: B-48, C III, and also cholesterol and triglyceride remnants which results in significant reduction of cardiovascular complication risk factors. In fact the administration of Liraglutide leads to a reduction of independent arterial sclerosis risk factors such as C-reactive protein (CRP) and brain natriuretic peptide (BNP). The decrease in their concentration inhibits the inflammatory cytokine activation directly engaged in vascular wall destruction. GLP-1 analogues may influence the early stages of aterogenesis by inhibition of monocyte adhesion to the vascular wall. . The use of liraglutide was related to TNF alfa inhibition and hyperglycemia-dependant gene PAI-1, ICAM-1, VCAM-1 induction in the cultures of human endothelial cells. Molecular mechanism of this process has not been explained, maybe Liraglutide may regulate expression of mRNA of NUR77 protein. This signal pathway seems to play a key role in endothelial cells protection in type 2 diabetes and in metabolic syndrome. At the same time, observed increase in the production of nitric oxide (NO) and inhibition of NF-κB partially by AMPK (AMP activate protein kinase), which documents anti-inflammatory influence of liraglutide on vascular endothelium cells even in hyperglycemia. The effect mentioned above can explain observed earlier vasodilative properties of the medication and its beneficial influence on cardiovascular system in patients suffering from type 2 diabetes.
Cardioprotective effect of liraglutide in comparison to metformin was showed in induced myocardial infarction in animals with diabetes, when similar metabolic control was achieved. This may be explained by liraglutide action on the increase of cAMP concentration and decrease of caspase 3 activation responsible for the cardiomyocyte apoptosis induction. This process was dependent on the GLP-1 receptor.
The influence of incretin mimetics on cardiovascular risk factors in diabetes,2012

RISKS OF TREATMENT WITH LIRAGLUTIDE
Post-marketing surveillance has identified sporadic cases of acute pancreatitis with both exenatide and sitagliptin; however, it is recognized that the incidence of pancreatitis is increased in patients with type 2 diabetes compared with the general population; there are several other risk factors that are also associated with an increased incidence of pancreatitis such as excess alcohol use and obesity. According to the 2011 liraglutide label there have been seven cases of pancreatitis (including two chronic cases) in liraglutide- treated patients , which is consistent with the higher incidence in the type 2 diabetes population. As with other incretin-based therapies, it is not possible to determine whether there is a link between pancreatitis and liraglutide because of the small number of cases observed.
Small increases in heart rate of between 2 and 4 beats per minute have been observed with liraglutide treatment in the LEAD studies. However, phase 1 studies of liraglutide showed no clinically relevant changes in the QTc interval on electrocardiogram. While cardiovascular risk was not specifically reported in the LEAD studies (other than blood pressure data), liraglutide treatment has been shown in a meta-analysis of the six LEAD studies to improve biomarkers of cardiovascular risk (high-sensitivity C-reactive protein and brain natriuretic peptide). The recently started LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) study aims to assess and confirm the cardiovascular safety of liraglutide, and investigate the potential for improved cardiovascular outcome. Three other large trials (ACCORD [Action to Control Cardiovascular Risk in Diabetes], ADVANCE [Action in Diabetes and Vascular Disease-PreterAx and DiamicroN Controlled Evaluation] and VADT [Veterans Affairs Diabetes Trial]) found no significant reduction in cardiovascular disease following intensive glycemic control.
GLP-1 and GLP-1 receptor agonists stimulate calcitonin release in rodents ; however, this effect is specific to rodents and has not been observed in primates including humans.
In agreement with the FDA, is not recommended special surveillance in liraglutide treated individuals. Unstimulated serum calcitonin levels were measured at 3-month intervals for up to 2 years in 5002 patients receiving liraglutide or control therapy in the LEAD program. This sequential screening showed that calcitonin levels were low and did not change over time. These data do not suggest any significant risk for activation of C-cells in humans in response to liraglutide.
The FDA has included a boxed warning on liraglutide’s label stating: ‘Liraglutide causes thyroid C-cell tumors at clinically relevant exposures in rodents. It is unknown whether Victoza causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as human relevance could not be determined by clinical or nonclinical studies. Victoza is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2’
An overview of the pharmacokinetics, efficacy and safety of liraglutide, 2011