Kraits snakes venom: bungarotoxins and their effects on human body

Bungarus, commonly referred to as kraits (pronounced kra-its), is a genus of venomous elapid snakes found in South and Southeast Asia. There are 13 species and five subspecies (excluding nominal) recognized.
Kraits are found in the Indian Subcontinent (including Sri Lanka and Bangladesh) and Southeast Asia (including Indonesia and Borneo).
The common krait's venom consists mainly of powerful neurotoxins, which induce muscle paralysis. Analyzing we can see that this venom contains presynaptic and postsynaptic neurotoxins,which generally affect the nerve endings near the synaptic cleft of the brain.
Incidents occur mainly at night because kraits are nocturnal. Frequently, little or no pain occurs from a krait bite (Krait's bites are significant for inducing minimal amounts of local inflammation/swelling), and this can give false reassurance to the victim. Typical symptoms after a krait-bite are, severe abdominal cramps, accompanied by progressive paralysis. As no local symptoms present, a patient should be carefully observed for signs of paralysis (the onset of ptosis) and treated urgently with antivenom. It's also possible to assist bittens by mechanical ventilation, using equipment available at hospitals. If death occurs, it takes place about four to eight hours after the bite. Cause of death is general respiratory failure, suffocation.
The few symptoms of having been bitten are: the facial muscles get tight in one to two hours, the patient may be unable to talk or see, and, if left untreated, the patient may die from respiratory paralysis within four to five hours. A clinical toxicology study gives an untreated mortality rate of 70-80%.
Common Krait, Wikipedia
A number of neurotoxins have been described in recent years, that may prove useful as specific pharmacological tools to study the mechanisms of synaptic transmitter release:
one source of such toxins, the venom of the Kraits, contains at least two types of neurotoxins.
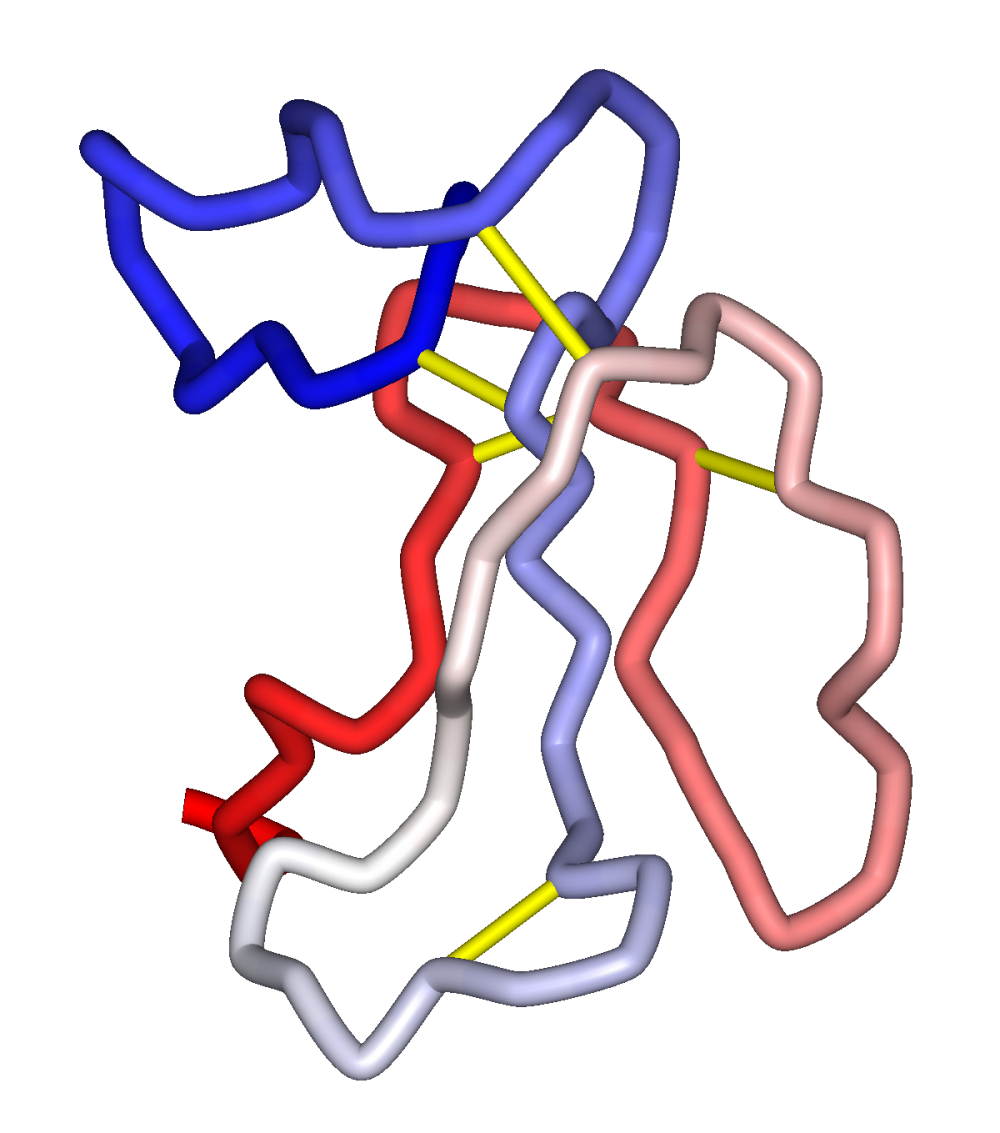
When the proteins of the crude venom are separated by column chromatography, postsynaptic or a-bungarotoxin (a-BuTX) are isolated, and presynaptic or b-bungarotoxin.
ALPHA - BUNGAROTOXIN
The nicotinic acetylcholine receptor (nAChR) carries two binding sites for snake's venom neurotoxins.
a-Bungarotoxin from the Southeast Asian Krait, is a long neurotoxin which competitively blocks AChR at the acetylcholine binding sites in a relatively irreversible manner.

Sites of action of snake neurotoxins and other substances on the neuromuscular junction. Schematic representation of the neuromuscular junction showing different sites of action of snake neurotoxins, other toxins, and pharmacological substances. 1. Synaptic vesicular proteins: Snake toxins: beta-bungarotoxin; Other toxins: botulinum toxin, tetanus neurotoxin. 2. Voltage-gated calcium channel: Snake toxins: beta- bungaratoxin ;3. Pre-synaptic membrane: Snake toxins: phospholipase A2 toxins. 4. Pre-synaptic ACh receptor ;
5. Voltage-gated potassium channels; 6. Acetylcholine: Lysis by exogenous acetylcholinesterase in snake venom. 7. Acetylcholinesterase: Inhibitors of endogenous AChE in snake venom. 8. Post-synaptic ACh receptors: Snake toxins: alpha-bungaratoxin; 9. Voltage-gated sodium channels.
The nicotinic acetylcholine receptor (nAChR) is a ligand-gated ion channel (LGIC) found in the neuromuscular junction of vertebrates.
The nAChR in the electrocyte membranes of the electric marine ray, Torpedo is the best characterized member of a superfamily of LGICs involved in information transfer in the brain and neuromusculature.
The nAChR is a large complex of four transmembrane glycoprotein subunits which form an a2bgd pentameric complex surrounding a central cation-conducting pore.
In native, fully-hydrated nAChR membranes, a-bungarotoxin binds to the nAChR outer vestibule and contacts the surface of the membrane bilayer.

The nAChR carries two binding sites for agonists and competitive antagonists and a single binding site for noncompetitive blockers. Acetylcholine is the natural agonist at nicotinic synapses, and the two a -subunits per receptor complex contain the agonist binding sites. Polypeptide neurotoxins act as competitive antagonists and include the venoms from snakes of the Elapidae (cobras, kraits, mambas, coral snakes, etc.) and Hydrophidae (sea snakes) families.
Our understanding of a-toxin binding has progressed rapidly with the crystal structure of the acetylcholine binding protein (AChBP) and numerous a-toxin structures by x-ray crystallography and NMR.
The AChBP is a homolog of the extracellular domain of nAChR and the crystal structure has allowed interpretation of the electron cryomicroscopy structure of nAChR a-Toxin has been shown to bind to the a-subunit between residues 172-205.
a-Bungarotoxin Binding to Acetylcholine Receptor Membranes Studied by Low Angle X-Ray Diffraction, 2003 Howard S. Young, Leo G. Herbette, and Victor Skita; Biophysical Journal Volume 85 .
BETA BUNGAROTOXIN

Beta- bungarotoxin, a neurotoxic single polypeptide with a molecular weight of approximately 11,000 and A2 phospholipase action, rappresents the main fraction of the venom of kraits.
It causes the failure of the mechanical response of the indirectly stimulated rat diaphragm, while the exposure to beta-bungarotoxin had no effect on the response of the muscle to direct stimulation. Resting membrane potentials of muscle fibres exposed to the toxin were similar to control values, and the binding of FITC-labelled alpha-bungarotoxin to nAChR at the neuromuscular junction was unchanged. Motor nerve terminal boutons at a third of cell junctions are destroyed by exposure to beta-bungarotoxin leaving only a synaptic gutter filled with Schwann cell processes and debris; at other junctions, some or all boutons survive exposure to the toxin. Synaptic vesicle density in surviving terminal boutons is reduced by 80% and synaptophysin immunoreactivity by >60% in preparations exposed to beta-bungarotoxin, but syntaxin and SNAP-25 immunoreactivity is largely unchanged. Terminal bouton area is also unchanged. Was seen that the depletion of synaptic vesicles is completely prevented by prior exposure to botulinum toxin C and significantly reduced by prior exposure to conotoxin. The data suggest that synaptic vesicle depletion is caused primarily by a toxin-induced entry of Ca(2+) into motor nerve terminals via voltage gated Ca(2+) channels and an enhanced exocytosis via the formation of t- and v-SNARE complexes.
Beta-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction, 2004 Prasarnpun S, Walsh J, Harris JB.
When the beta bungarotoxin is inoculated into one hind limb of young adult rats it is paralysed within 3 h, and remaines paralysed for 2 days.
The paralysis is associated with the loss of synaptic vesicles from motor nerve terminal boutons, a decline in immunoreactivity of synaptophysin, SNAP-25 and syntaxin, a loss of muscle mass and the upregulation of NaV1.5mRNA and protein. Between 3 and 6 h after the inoculation of toxin, some nerve terminal boutons exhibite clear signs of degeneration; others appeare to be in the process of withdrawing from the synaptic cleft and some boutons are fully enwrapped in terminal Schwann cell processes. By 12 h all muscle fibres are denervated. Re-innervation begin at 3 days with the appearance of regenerating nerve terminals, a return of neuromuscular function in some muscles and a progressive increase in the immunoreactivityof synaptophysin, SNAP-25 and syntaxin. Full recovery occur at 7 days. The data are compared with recently published clinical data on envenoming bites by kraits and by extrapolation we suggest that the acute, reversible denervation caused by b-bungarotoxin is a credible explanation for the clinically important,profound treatment-resistant neuromuscular paralysis seen in human subjects bitten by these animals.
From Envenoming bites by kraits: the biological basis of treatment-resistant neuromuscular paralysis S. Prasarnpun,1 J. Walsh, S. S. Awad and J. B. Harrish; 2005
 Fig. 1 Representative images of NMJs in soleus muscle fibres at various times after the inoculation of β-bungarotoxin (2.0 µg). Control terminals (A) were full of synaptic vesicles (arrow head) and mitochondria (M). At 3 h terminals contained reduced numbers of synaptic vesicles and damaged mitochondria (B and C). Schwann cell processes had often invaded the synaptic cleft between post-junctional trough and terminal bouton (P in B) and terminals appeared to be withdrawing from the synaptic troughs (SC in C). At 24 h (D) most synaptic troughs were empty but retraction bulbs (RB) were common. The bulb shown in D remained attached to the post-synaptic basal lamina (*). Scale bar 1 µm (B–D) or 2 µm (A).
Fig. 1 Representative images of NMJs in soleus muscle fibres at various times after the inoculation of β-bungarotoxin (2.0 µg). Control terminals (A) were full of synaptic vesicles (arrow head) and mitochondria (M). At 3 h terminals contained reduced numbers of synaptic vesicles and damaged mitochondria (B and C). Schwann cell processes had often invaded the synaptic cleft between post-junctional trough and terminal bouton (P in B) and terminals appeared to be withdrawing from the synaptic troughs (SC in C). At 24 h (D) most synaptic troughs were empty but retraction bulbs (RB) were common. The bulb shown in D remained attached to the post-synaptic basal lamina (*). Scale bar 1 µm (B–D) or 2 µm (A).
 Fig. 2 Representative images of NMJs in soleus muscle fibres 2 days (A), 3 days (B) and 5 days © after the inoculation of β-bungarotoxin (2.0 µg). At 2 days synaptic troughs contained invading pseudopodia-like processes (P in A). These processes were devoid of basal lamina or other specialized structures. Their identity was unclear. By 3 days small nerve terminals (NT in B) were seen in ∼60% of junctions. By 5 days small nerve terminals were seen in all junctions and attachments were identified between new nerve terminals and the post-synaptic basal lamina (* in images B and C). Scale bar: 1 µm in all images.
Fig. 2 Representative images of NMJs in soleus muscle fibres 2 days (A), 3 days (B) and 5 days © after the inoculation of β-bungarotoxin (2.0 µg). At 2 days synaptic troughs contained invading pseudopodia-like processes (P in A). These processes were devoid of basal lamina or other specialized structures. Their identity was unclear. By 3 days small nerve terminals (NT in B) were seen in ∼60% of junctions. By 5 days small nerve terminals were seen in all junctions and attachments were identified between new nerve terminals and the post-synaptic basal lamina (* in images B and C). Scale bar: 1 µm in all images.
The application of the enzymatically active toxin to the frog sciatic nerve-sartorius muscle preparation results in an +initial decrease in the average endplate potential amplitude followed by a temporary rebound in endplate potential amplitude*, and finally a complete inhibition of endplate potentials.
Similarly, miniature endplate potential frequency is initially reduced upon toxin application but then increases dramatically. After the phospholipaseA2 of the toxin is inactivated, treatment with the toxin results in only the initial decrease in transmitter release. These results suggest that this B-bungarotoxin acts in two functionally separate steps:
- by binding to a specific presynaptic site possibly associated with calcium entry, and
- by perturbing the presynaptic membrane by its enzyme action, which results in an increase and then a failure in transmitter release.
Some author have postulated that the PLA activity is responsible for the toxicity of this protein. However, other have suggested that the phospholipase is a portion of the toxin molecule that is separate from the component responsible for specific presynaptic binding.
Blockade of neuromuscular transmission by enzymatically active and inactive fl-bungarotoxin; 1976
David R. Livengood, Richard S. Mannalist, Mildred A. Donnolon, Leona M. Masukawa,
Gene S. Tobias, and William Shain;

CLINICAL CASES : deep coma and hypokalaemia
Bungarus caeruleus (Common Krait) bites are an important medical problem in South Asia.
Krait's bite rapidly lead to a typical occulo-facio-bulbar and respiratory muscle paralysis that needs mechanical ventilation in 50% of all bitten.

Physicians managing Kraits bites frequently observe patients in states of deep coma mimicking brain death. They reported a state of total unresponsiveness to deep pain or loud voices associated with fixed dilated pupils and absent brain stem and spinal reflexes. Often survivors made a complete neurological recovery after different periods of mechanical ventilation. This state of deep coma may be due to hypoxia secondary to respiratory failure or a direct effect of venom on the cortical and/or brainstem neurons.
Authors assumed that EEG and brainstem auditory and visual evoked potentials will be abnormal if the venom acted on the cortical and brainstem neurones.
Another intriguing phenomenon reported following Kraits bite in Sri Lanka is high incidence (71%) of hypokalaemia (<3.5mmol/l). Beta adrenergic stimulation and intra-cellular shift of potassium are considered as the potential cause of this phenomenon. A third possibility is loss of potassium in the renal tubules. Is postulated that measurement of urinary potassium excretion would be a +reliable surrogate marker of renal conservation of potassium*. If there is intracellular shifting of potassium, the renal tubules would attempt to conserve potassium and thus, potassium excretion of would be minimal.
 Let’s talk about two clinical cases.
Let’s talk about two clinical cases.
Two patients were presented to the local hospital with the killed specimen of Bungarus caeruleus.
Both patients were treated with polyvalent antivenom (20 vials of Lyophilised, Enzyme refined, Equine Snake Venom Antiserum; (Vins Bioproducts Limited® ) raised against Indian Cobra, Common Krait, Russell's Viper and Saw Scaled Viper) at the local hospital, before being transferred to the study hospital for further care.
Patient 1
He arrived with a normal Glasgow Coma Scale (GCS), peripheral oxygen saturation of 100% and normal blood pressure. Within an hour after admission, he developed respiratory failure and was intubated. On arrival at the study hospital, his GCS was 3/15, Blood pressure 90/70 mmHg and pulse rate of 80/min.
He did not respond to loud commands or deep pain. His pupils were dilated (6mm) and were not reacting to light. All brain stem and spinal reflexes were absent. Metabolic acidosis was seen.
Over the next 4 days, his cortical functions improved from a flicker of movement of the eye lids to deep pain to movement of hands to verbal commands (GCS 10/15). On day 7, he developed features of disseminated intravascular coagulation (DIC), X ray revealed evidence of Adult Respiratory Distress Syndrome (ARDS). Maintenance of oxygenation became increasingly difficult and he died of an asystolic cardiac arrest 13 days after the bite. Post-mortem examination revealed macroscopic evidence of ARDS. Histology of the diaphragm revealed evidence of extensive muscle necrosis.
 Hematoxylin & Eosin section of diaphragmatic muscle. Arrow indicates an area of necrosis.
Hematoxylin & Eosin section of diaphragmatic muscle. Arrow indicates an area of necrosis.
Serum Potassium was found to be persistently low (2.2, 2.1, 3.1, 3 and 3mmol/l on admission, days 2,3,4 and 5 respectively) despite replacement of potassium at a rate of 1-1.5mmol/kg/day. Twenty four hour urinary potassium excretion was persistently lower than the normal 1-1.5mmol/kg/day.
 Daily serum potassium and urinary potassium excretion. In patient 1, serum potassium remained below normal (3.5mmol/l) throughout while urinary potassium excretion remained below normal daily excretion (1-1.5mmol/kg/day). In patient 2, serum potassium normalized on day 3 while the urinary excretion remained low.
Daily serum potassium and urinary potassium excretion. In patient 1, serum potassium remained below normal (3.5mmol/l) throughout while urinary potassium excretion remained below normal daily excretion (1-1.5mmol/kg/day). In patient 2, serum potassium normalized on day 3 while the urinary excretion remained low.
EEG revealed absent alpha and delta activity and dominant theta activity (4-5Hz) on the day of the bite. Serial daily EEGs revealed that alpha activity and delta did not appear while theta activity dominated for 7 days after the bite.
 EEG of patient 1 done on the day of the bite demonstrating absent alpha and delta activity with dominant theta activity.
EEG of patient 1 done on the day of the bite demonstrating absent alpha and delta activity with dominant theta activity.
 Delayed brainstem auditory evoked potentials to click stimulus in Patient 1. Top trace recorded from A2-Fz and bottom trace from A1-Fz.
Delayed brainstem auditory evoked potentials to click stimulus in Patient 1. Top trace recorded from A2-Fz and bottom trace from A1-Fz.
Patient 2
He developed respiratory paralysis 10hr after the bite and was intubated. He had normal cardiovascular functions throughout the illness. He has a GCS of 3/15 with no brain stem and spinal reflexes. On the 3rd day after the bite, he regained consciousness and was extubated on the 4th day and discharged with no residual neurological complications. He had no memory of events that occurred after the bite until the third day.
His serum potassium was low (2.9mmol/L) on the day of the admission and improved (3.8mmol/L)on day 3. EEG on day 1 revealed theta activity with absent alpha and delta activity for 2 days. Alpha activity returned on day 3.
 EEG of patient 2 done on the day of the bite demonstrating theta activity with absent alpha and delta activity.
EEG of patient 2 done on the day of the bite demonstrating theta activity with absent alpha and delta activity.
Severely envenomed patients with Bungarus caeruleus have depressed cortical activity and brain stem functions. In addition to deep coma, both patients had identical EEG activity of absent alpha and delta waves and dominant theta waves after the bite. The survivor had restored alpha activity on the third day while in the other, alpha activity did not return.
Both patients demonstrated hypokalaemia with reduced excretion of urinary potassium. The cause of death in the Patient 1 was a combination of disseminated intravascular coagulopathy and adult respiratory distress syndrome (ARDS).
Deep coma and hypokalaemia of unknown aetiology following Bungarus caeruleus bites: Exploration of pathophysiological mechanisms with two case studies; 2010 Indika Bandara Gawarammana, Senanayake Abeysinghe Mudiyanselage Kularatne,Keerthi Kularatne, Roshita Waduge, Vajira Senaka Weerasinghe, Sunil Bowatta,and Nimal Senanayake;