DEFINITION
Sjögren’s syndrome is a chronic inflammatory and autoimmune disease in which the salivary and lacrimal glands undergo progressive destruction by lymphocytes and plasma cells resulting in decreased production of saliva and tears.
Primary Sjögren syndrome occurs in the absence of another underlying rheumatic disorder, whereas secondary Sjögren syndrome is associated with another underlying rheumatic disease, such as systemic Lupus erythematosus (SLE), Rheumatoid Arthritis (RA), or Scleroderma. Given the overlap of Sjögren syndrome with many other rheumatic disorders, it is sometimes difficult to determine whether a clinical manifestation is solely a consequence of Sjögren syndrome or is due to one of its overlapping disorders.
It is named after Swedish ophthalmologist Henrik Sjögren (1899–1986) who first described it.
EPIDEMIOLOGY
This condition can affect people of all races and any age, but symptoms usually appear in the fourth to fifth decade of life. The female-to-male ratio is 9:1.
About half of affected patients also have rheumatoid arthritis or other connective tissue deseases, such as lupus.
As shown in the comprehensive table, from the article: The geoepidemiology of Sjögren's syndrome by Mavragani CP, Moutsopoulos HM,
there is a widely reported range between different studies varying from 0.1 to 4.8%.
The observed variability might reflect differences in the definition and application of diagnostic criteria between the studies, the diverse geographic study origin (implying the contribution of both genetic and environmental factors) as well as the different initial sample size and sex distribution.

SYMPTOMS
The initial manifestations may be nonspecific and insidious, such as dry eyes and dry mouth, musculoskeletal involvement (60%), fatigue (60%), arthralgias (60-70%), myalgias (20-30%), dry skin (40%), vaginal dryness (40%).
The typical presentation is known as the “sicca complex”, which symptoms are:
• Dry eyes.
Dry eyes may be described as red, itchy, and painful. However, the most typical complaint is that of a gritty or sandy sensation in the eyes. Symptoms typically worsen throughout the day, probably due to evaporation of the already scanty aqueous layer. Others have trouble with blurry vision, or are bothered by bright light, especially fluorescent lighting. Some patients awaken with matting in their eyes and, when severe, have difficulty opening their eyes in the morning. These symptoms can result in the destruction of corneal and bulbar conjunctival epithelium defined as keratoconjunctivitis sicca.
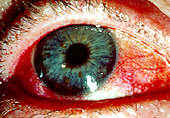
• Dry mouth.
Dry mouth is frequent but variable in severity.
Patients may describe dry mouth in various ways, as follows:
- Inability to eat dry food (eg, crackers) because it sticks to the roof the mouth
- Tongue sticking to the roof of the mouth
- Putting a glass of water on their bed stand to drink at night (and resulting nocturia)
- Difficulty speaking for long periods of time or the development of hoarseness
- Higher incidence of dental caries and periodontal disease
- Altered sense of taste
- Difficulty wearing dentures
- Development of oral candidiasis with angular cheilitis, which can cause mouth pain

• Patients may also have difficulty with dry skin and a dry vagina that can lead to dyspareunia, vaginitis, and pruritus.
• Nasal dryness can result in discomfort and bleeding
Other manifestations are:
- Parotitis
Patients with Sjögren syndrome may have a history of recurrent parotitis, often bilateral.
Some patients have parotid glands large enough that they report this as a problem. More often, the examining physician discovers them.
- Extraglandular (systemic) involvement
- Skin and related symptoms
- Cutaneous vasculitis such as palpable purpura develops in some patients with Sjögren
syndrome, especially those with hypergammaglobulinemia or cryoglobulinemia.
- Raynaud phenomenon is observed in approximately 20% of patients.
- Pulmonary symptoms
- Patients with Sjögren syndrome can develop dryness of the tracheobronchial mucosa (xerotrachea), which can manifest as a dry cough.
- Patients may develop recurrent bronchitis or even pneumonitis (infectious or noninfectious).
- Gastrointestinal symptoms
- Dryness of the pharynx and esophagus frequently leads to difficulty with swallowing (deglutition), in which patients usually describe food becoming stuck in the upper throat.
- Lack of saliva may lead to impaired clearance of acid and may result in gastroesophageal reflux and esophagitis.
- Abdominal pain and diarrhea can occur.
- Cardiac symptoms
- Pericarditis and pulmonary hypertension, with their attendant symptomatology, can occur in Sjögren syndrome.
- Orthostatic symptoms related to dysfunction of autonomic control of blood pressure and heart rate is associated with increased severity of Sjögren syndrome
- Neurologic symptoms
- The occurrence of central nervous system and spinal cord involvement in Sjögren syndrome is estimated by various studies at 8-40%, with manifestations including myelopathy, optic neuropathy, seizures, cognitive dysfunction, and encephalopathy. Attempts must be made to distinguish other causes of these symptoms, including concomitant SLE, multiple sclerosis, cerebrovascular disease, and Alzheimer disease.
- Sensory, motor, or sensory-motor peripheral neuropathy, often subclinical, can be detected in up to 55% of unselected patients with Sjögren syndrome. Symptoms of distal paresthesias may be present.
- Renal symptoms
- Renal calculi, renal tubular acidosis, and osteomalacia can occur secondary to tubular damage caused by interstitial nephritis, the most common form of renal involvement in Sjögren syndrome.
- Interstitial cystitis, with symptoms of dysuria, frequency, urgency, and nocturia, is strongly associated with Sjögren syndrome.
- Glomerulonephritis can be caused by Sjögren syndrome but is uncommon and is usually attributable to another disorder such as SLE or mixed cryoglobulinemia.
- Other symptoms
- Patients with Sjögren syndrome may report fatigue, joint pain, and, sometimes, joint swelling.
- Women with Sjögren syndrome may have a history of recurrent miscarriages or stillbirths, and both women and men may have a history of venous or arterial thrombosis. These are related to the presence of antiphospholipid antibodies (eg, lupus anticoagulant or anticardiolipin antibodies).
DIAGNOSIS
Classification criteria
According to the American-European Consensus, diagnosis of primary Sjögren syndrome requires 4 of 6 of the below criteria. Sjögren syndrome can be diagnosed in patients who have no sicca symptoms if 3 of 4 objective criteria are fulfilled.
The criteria are as follows:
- Ocular symptoms
- Dry eyes for more than 3 months
- Foreign-body sensation
- Use of tear substitutes more than 3 times per day
- Oral symptoms
- Feeling of dry mouth
- Recurrently swollen salivary glands
- Frequent use of liquids to aid swallowing
- Ocular signs
- Schirmer test performed without anesthesia to measure tear production (<5 mm in 5 min)
- Positive vital dye staining results, like rose bengal and lissamine green. (Eyedrops containing dyes to examine the surface of the eye)
- Oral signs
- Abnormal salivary scintigraphy findings. A nuclear medicine test that measures salivary gland function reduction.
- Abnormal parotid sialography findings. This study is interpreted by evaluating the morphology of the salivary ducts for obstructions and chronic inflammation.
- Abnormal sialometry findings (unstimulated salivary flow <1.5 mL in 15 minutes)
- Positive minor salivary gland biopsy findings (usually in the lower lip).
It confirms inflammatory cell (lymphocytic) infiltration of the minor salivary glands. More than 1 focal aggregate is seen per 4 mm2. Focal aggregates = at least 50 lymphocytes.
- Positive anti–SSA or anti–SSB antibody results
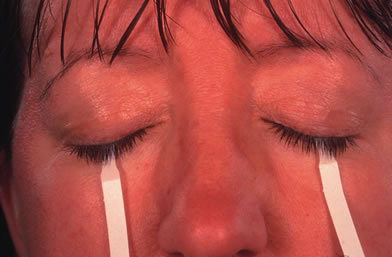
Schirmer's test

Biopsy
Secondary Sjögren syndrome is diagnosed when, in the presence of a connective-tissue disease, symptoms of oral or ocular dryness are present in addition to criterion 3, 4, or 5 above.
Application of these criteria has yielded a sensitivity of 97.2% and a specificity of 48.6% for the diagnosis of primary Sjögren syndrome; for secondary Sjögren syndrome, the specificity was 97.2% and the sensitivity 64.7%.
Blood test can also be useful to evidence other anormalities such as:
- Polyclonal Gammopathy
- Antithyroid Antibodies
- Rheumatoid Factors (RF) (52%)
- Antinuclear Antibodies (ANAs)
- Creatinine Clearance Diminished (50%)
- Normochromic Normocytic Anemia (50%)
- Leukopenia (42%)
- Sedimention Rate Elevated (80%)
PATHOGENESIS
Recent discoveries from studies in patients with Sjögren's syndrome and animal models suggest a complex interplay between genetic factors, environmental events that involve innate and adaptive immunity, hormonal mechanisms and the ANS.
Genetic factors
HLA class II markers confer genetic susceptibility to Sjögren's syndrome.
The genetic associations in Sjögren syndrome vary among ethnic groups. In white persons, for instance, the condition is linked to human leukocyte antigen (HLA)–DR3, HLA-DQ2, and HLA-B8,whereas the linkage is to HLA-DRB1*15 in Spanish persons and to HLA-DR5 in Greek and Israeli persons.
Gottenberg et al. (2003) compared 149 patients fulfilling the American-European Consensus Group criteria for Sjogren syndrome to 222 controls and confirmed the association of Sjogren syndrome with HLA alleles DRB1*03 (see HLA-DRB1; 142857) and DQB1*02 (see HLA-DQB1; 604305). They found, however, that the association between HLA and SS is restricted to patients with anti-SSA and/or anti-SSB antibodies; HLA is not associated with SS in patients without these autoantibodies. The absence of a difference in disease severity between patients with anti-SSA and those with anti-SSA and anti-SSB antibodies, together with a high frequency of HLA-DRB1*03 in the latter group, suggested to the authors that HLA alleles predispose to autoantibody secretion but are not associated with clinical out come.
Viral factors
Evidence has accumulated on the associations of the etiology and several viruses such as HIV retrovirus, HTLV-1, hepatitis virus and Epstein-Barr virus (EBV). Among these viruses, EBV is a strong candidate for the cause of this disease since the EBV is an ubiquity in humans and the
EBV DNA is detected in substantial proportion of epithelial cells.
The virus stimulates the immune system to act sending lymphocytes to the glands of the eyes and mouth. Once there, the lymphocytes attack healthy cells, causing the inflammation that damages the glands and keeps them from working properly. These cells are supposed to die with apoptosis, but in Sjögren’s syndrome, they continue to attack, causing further damage.
Sjögren's syndrome. Yes autoreactive lymphocytes, why? Virus or gene?
Hormonal factors
Literature analysis suggested androgen dependency of human CRISP-3(cysteine-rich secretory protein 3) and this was verified by studies of human submandibular gland acinar cells cultured with or without DHEA, in which DHEA increased CRISP-3 messenger RNA (mRNA) levels.
Compared with healthy controls, SS patients had low serum levels of DHEAS (P = 0.008) and also low salivary levels of DHEA.
CRISP-3 pathology was seen in acini, where it has lost its polarized organization, even in regions without inflammatory cells, and so it may represent some systemic effect in SS.
Low salivary dehydroepiandrosterone and androgen-regulated cysteine-rich secretory protein 3 levels in Sjögren's syndrome
Autonomic nervous system
Autonomic nervous system (ANS) abnormalities are common in Sjögren's syndrome and may play an etiologic role in its pathogenesis. The vascularity and secretory function of exocrine glands affected in Sjögren's syndrome are innervated by the sympathetic and parasympathetic branches of the ANS. Sjögren's syndrome mimics several ANS failure syndromes. Xerostomia and xerophthalmia, the cardinal Sjögren's syndrome manifestations, are features of cholinergic parasympathetic ANS dysfunction, whereas sympathetic cholinergic failure results in xerosis and decreased sweating that are frequently reported by Sjögren's syndrome patients. Fatigue, another prominent feature of Sjögren's syndrome, has also been associated with ANS dysfunction.
The complexity of the ANS along with differences in methodology and studied populations has resulted in variable results, but abnormalities in Sjögren's syndrome have been reported both in sympathetic and parasympathetic ANS domains with prevalence as high as 90%.
Antimuscarinic receptor autoantibodies
The major stimulus for saliva production is provided by acetylcholine through muscarinic acetylcholine receptors of which the type 3 receptor [muscarinic 3 receptor (M3R)] is responsible for saliva production. The description of autoantibodies against the M3R generated a lot of interest and controversies over the last decade. Some groups have found anti-M3R receptors in up to 90% of Sjögren's patients using peptide ELISAs, whereas others were unable to detect it by immunological methods.
Immune system abnormalities
It is increasingly recognized that the innate immune system plays a crucial rule in the immunepathogenesis of Sjögren's syndrome.
Recent studies have focused on the central role of type-I IFNs and the more recently described B cell-activating factor (BAFF), which may represent a link between innate and adaptive immunity.
Enhanced activity of the type-1 IFN system has been linked to multiple autoimmune diseases, including Sjögren's syndrome. One of the cytokines upregulated by IFNα is BAFF.
Excessive production of BAFF could contribute to the pathogenesis of SjS by several mechanisms. First, although BAFF is not required for the initiation of a germinal centre, it has been found to play critical roles in sustaining or maintaining a germinal centre reaction as well as in establishing follicular dendritic cell networks. Thus, by binding to BAFF receptor, BAFF could contribute to the formation of ectopic germinal centres, and a mature follicular dendritic cell network that is capable of retaining immune complexes, in salivary glands. Second, BAFF is a well characterized survival factor for human plasmablasts generated from memory B cells. The predominance of memory B cells and activated T cells in the salivary glands, coupled with the increased serum levels of interleukin-10 in SjS patients, could provide an environment for the production of plasmablasts producing autoantibodies. Third, BAFF can co-stimulate proliferation and cytokine secretion by activated CD4+ T cells in a BAFF receptor dependent manner.
Animals models
It was also observed salivary gland dysfunction and abnormal distribution of aquaporin (AQP) 5, which plays an essential role in transcellular water transport in salivary glands. Similarly, basolateral misdistribution of AQP5 associated with decreased salivary function was reported in the submandibular glands, and AQP5-trafficking defects were also documented in lacrimal glands of nonobese diabetic (NOD) mice. Moreover, parasympathetic denervation of rat submandibular glands resulted in degradation of AQP5 that was rescued by administration of muscarinic receptor 3 selective agonist cevimeline. In the NOD mouse model of Sjögren's syndrome, salivary gland vasculature showed significantly decreased vasodilatation responses to parasympathetic stimulation, and muscarinic receptor activation partly attributed to decreased nitric oxide signalling. These data suggest that abnormalities of water and ion channels are not always caused by inflammation but may represent nonimmunologic mechanisms of Sjögren's syndrome exocrine dysfunction.
Pathogenesis of Sjögren's syndrome
COMPLICATIONS
Patients with Sjögren's syndrome have a higher rate of non-Hodgkin lymphoma compared to both patients with other autoimmune diseases and healthy people. About 5% of patients with Sjögren's syndrome will develop some form of lymphoid malignancy. Patients with severe cases are much more likely to develop lymphomas than patients with mild or moderate cases. The most common lymphomas are salivary extranodal marginal zone B cell lymphomas (MALT lymphomas in the salivary glands) and diffuse large B-cell lymphoma.
Persistent enlargement of a major salivary gland should be carefully and regularly observed and investigated further if it changes in size in a short period of time. Other symptoms may include the following:
- reddened patches on the skin.

THERAPY
There is no cure for Sjogren's syndrome and no treatment that can restore the ability of the glands to produce moisture. The goal of treatment is to relieve symptoms.
Treatments include:
- MEDICATION
- Artificial tears and vaginal lubricants can help relieve dryness. Restasis may be used to increase tear production.
- Dry mouth can be relieved by drinking water, chewing gum, or using saliva substitutes. Some patients benefit from using prescription medications that stimulate saliva flow, such as PILOCARPINE (Salagen) or CEVIMULINE (Evoxac).
- Steroids or NSAID to relieve inflammation and swelling.
- Hydroxychloroquine (Plaquinel), an antimalarial drug used in lupus and rheumatoid arthritis, may be helpful in some patients with Sjögren's syndrome by reducing joint pain and rash experienced by some patients. Patients with rare but serious systemic symptoms, such as fever, rashes, abdominal pain, or lung or kidney problems, may require treatment with corticosteroids such as PREDNISONE (Deltasone and others) and/or immunosuppressive agents METHOTREXATE (Rheumatrex), AZATHIOPRINE (Imuran), MYCOPHENOLATE (Cellcept), CYCLOPHOSPHAMIDE (Cytoxan).
- IGIV therapy, for severe, systemic or non responsive forms
- LIFESTYLE MEASURES
- Mild exercise can help relieve joint stiffness.
- Sipping liquids frequently and sucking on sugar-free candies can relieve dry mouth.
- Brushing, flossing, and seeing your dentist regularly can prevent cavities.
- Using nonscented moisturizers can help relieve dry skin.
Authors: Laura Tomasi Cont and Stella Campagnolo