Plant description
Tagetes erecta, the Mexican marigold, also called Aztec marigold, is a species of the genus Tagetes native to Mexico and Central America. Despite its being native to the Americas, it is often called African marigold. In Mexico, this plant is found in the wild in the states of San Luis Potosí, Chiapas, State of México, Puebla, Sinaloa, Tlaxcala, and Veracruz. This plant reaches heights of between 50–100 cm (20–39 in). The Aztecs gathered the wild plant as well as cultivating it for medicinal, ceremonial and decorative purposes. It is widely cultivated commercially with many cultivars in use as ornamental plants. The common name of marigold is also used for calendula.
Since prehispanic times, this plant has been used for medicinal purposes. The Cherokee used it as a skin wash and for yellow dye. Scientific study shows that thiophenes, natural phytochemicals that include sulfur-containing rings, may be the active ingredients. They have been shown to kill gram negative and gram positive bacteria in vitro. This marigold may help protect certain crop plants from nematode pests when planted in fields. It is most effective against the nematode species Pratylenchus penetrans. Today, T. erecta is grown to extract lutein, a common yellow/orange food colour (E161b). The essential oil of the flower contains antioxidants.

Scientific classification
Kingdom: Plantae
(unranked): Angiosperms
(unranked): Eudicots
(unranked): Asterids
Order: Asterales
Family: Asteraceae
Genus: Tagetes
Species: T. erecta
Binomial name: Tagetes erecta L.
Lutein
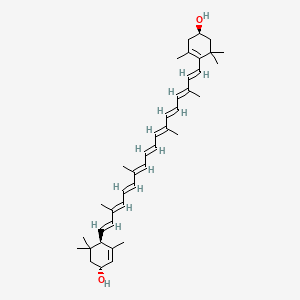
Also known as: Lutein ester, Vegetable lutein, Vegetable luteol, all-trans-Lutein, all-trans-(+)-Xanthophyll, Luteine, Lutein, all-trans-
Molecular Formula: C40H56O2
Molecular Weight: 568.87144
It is a carotenoid alcohol widespread in nature. It is present in egg yolk, algae, and petals of yellow flowers, among other sources.
Carotenoids, which are synthesized de novo by microorganisms and plants, accumulate in various biological tissues throughout the food chain. More than 700 carotenoids, including the metabolites in animals, are present in nature. Most of the carotenoids contain oxygen functions in the molecules, and these carotenoids are referred to as xanthophylls. In recent years, a great deal of attention has been focused on biological activities of dietary xanthophylls such as lutein, zeaxanthin, β-cryptoxanthin, capsanthin, astaxanthin, and fucoxanthin.
Lutein is one of the major xanthophylls present in green leafy vegetables. Lutein and zeaxanthin are known to selectively accumulate in the macula of the human retina. The latter predominates at the macula lutea while lutein predominates elsewhere in the retina. They have been thought to work as antioxidants and as blue light filters from the ionizing effect of blue light to protect the eyes from such oxidative stresses as cigarette smoking and sunlight exposure, which can lead to age-related macular degeneration and cataracts.
Lutein is obtained by animals directly or indirectly, from plants. In addition to coloring yolks, lutein causes the yellow color of chicken skin and fat, and is used in chicken feed for this purpose.
The principal natural stereoisomer of lutein is (3R,3′R,6′R)-beta,epsilon-carotene-3,3′-diol. Lutein is a lipophilic molecule and is generally insoluble in water. The presence of the long chromophore of conjugated double bonds (polyene chain) provides the distinctive light-absorbing properties. The polyene chain is susceptible to oxidative degradation by light or heat and is chemically unstable in acids. Lutein is present in plants as fatty-acid esters, with one or two fatty acids bound to the two hydroxyl-groups. For this reason, saponification (de-esterfication) of lutein esters to yield free lutein may yield lutein in any ratio from 1:1 to 1:2 molar ratio with the saponifying fatty acid.
Several proposed metabolites of lutein, were previously known to be present in such human tissues as plasma, milk, liver, and retina. Moreover, we found a remarkable accumulation of metabolites in mice fed with lutein. 3′-Hydroxy-ɛ,ɛ-caroten-3-one and lutein were the predominant carotenoids in the plasma, liver, kidney, and adipose, accompanied by ɛ,ɛ-carotene-3,3′-dione, indicating that mice actively convert lutein to keto-carotenoids by oxidizing the secondary hydroxyl group. However, 3-hydroxy-β,ɛ-caroten-3′-one (3′-oxolutein), the major metabolite of lutein in human plasma and the retina , was not detected in the tissues of the mice.

These metabolites would be formed by the same enzyme that mediated the conversion of fucoxanthinol to amarouciaxanthin A. The combined level of the lutein metabolites in the liver of the mice was 72.4% of the total (intact lutein and the metabolites). This indicates that quantification of the metabolites is necessary to estimate the lutein bioavailability. Moreover, intact lutein and the metabolites may differ in their biological activities. Differences among lutein and its metabolites as antioxidants and blue light filters deserved further study.
Structural aspects of the antioxidant activity of lutein in a model of photoreceptor membranes.
Source
Department of Biophysics, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Kraków, Poland.
Abstract
It was shown that in membranes containing raft domains, the macular xanthophylls lutein and zeaxanthin are not distributed uniformly, but are excluded from saturated raft domains and about ten times more concentrated in unsaturated bulk lipids. The selective accumulation of lutein and zeaxanthin in direct proximity to unsaturated lipids, which are especially susceptible to lipid peroxidation, could be very important as far as their antioxidant activity is concerned. Therefore, the protective role of lutein against lipid peroxidation was investigated in membranes made of raft-forming mixtures and in models of photoreceptor outer segment membranes and compared with their antioxidant activity in homogeneous membranes composed of unsaturated lipids. Lipid peroxidation was induced by photosensitized reactions using rose Bengal and monitored by an MDA-TBA test, an iodometric assay, and oxygen consumption (using EPR spectroscopy and the mHCTPO spin label as an oxygen probe).
 The results show that lutein protects unsaturated lipids more effectively in membranes made of raft-forming mixtures than in homogeneous membranes. This suggests that the selective accumulation of macular xanthophylls in the most vulnerable regions of photoreceptor membranes may play an important role in enhancing their antioxidant properties and ability to prevent age-related macular diseases (such as age-related macular degeneration [AMD]).
The results show that lutein protects unsaturated lipids more effectively in membranes made of raft-forming mixtures than in homogeneous membranes. This suggests that the selective accumulation of macular xanthophylls in the most vulnerable regions of photoreceptor membranes may play an important role in enhancing their antioxidant properties and ability to prevent age-related macular diseases (such as age-related macular degeneration [AMD]).
Effects of Lutein and Zeaxanthin Supplementation on Age-related Macular Degeneration
Interactions of dietary carotenoids with singlet oxygen ((1)O(2)) and free radicals: potential effects for human health.
Source
Department of Dermatology, Charité-Universitätsmedizin, Berlin, Germany.
Abstract
The dietary carotenoids provide photoprotection to photosynthetic organisms, the eye and the skin. The protection mechanisms involve both quenching of singlet oxygen and of damaging free radicals. The mechanisms for singlet oxygen quenching and protection against free radicals are quite different - indeed, under some conditions, quenching of free radicals can lead to a switch from a beneficial anti-oxidant process to damaging pro-oxidative situation. Furthermore, while skin protection involves β-carotene or lycopene from a tomato-rich diet, protection of the macula involves the hydroxyl-carotenoids (xanthophylls) zeaxanthin and lutein. Time resolved studies of singlet oxygen and free radicals and their interaction with carotenoids via pulsed laser and fast electron (pulse radiolysis) and the possible involvement of amino acids are discussed and used to (1) speculate on the anti- and pro-oxidative mechanisms, (2) determine the most efficient singlet oxygen quencher and (3) demonstrate the benefits to photoprotection of the eye from the xanthophylls rather than from hydrocarbon carotenoids such as β-carotene.
A naturally occurring carotenoid, lutein, reduces PDGF and H2O2 signaling and compromised migration in cultured vascular smooth muscle cells
Platelet-derived growth factor (PDGF) is a potent stimulator of growth and motility of vascular smooth muscle cells (VSMCs). Abnormalities of PDGF/PDGF receptor (PDGFR) are thought to contribute to vascular diseases and malignancy. We previously showed that a carotenoid, lycopene, can directly bind to PDGF and affect its related functions in VSMCs. In this study we examined the effect of the other naturally occurring carotenoid, lutein, on PDGF signaling and migration in VSMCs.
Lutein reduced PDGF signaling, including phosphorylation of PDGFR-β and its downstream protein kinases/enzymes such as phospholipase C-γ, Akt, and mitogen-activated protein kinases (MAPKs). Although lutein possesses a similar structure to lycopene, it was striking that lutein inhibited PDGF signaling through a different way from lycopene in VSMCs. Unlike lycopene, lutein not only interacted with (bound to) PDGF but also interfered with cellular components. This was evidenced that preincubation of PDGF with lutein and treatment of VSMCs with lutein followed by removing of lutein compromised PDGF-induced signaling. Lutein reduced PDGF-induced intracellular reactive oxygen species (ROS) production and attenuated ROS- (H2O2-) induced ERK1/2 and p38 MAPK activation. A further analysis indicated lutein could inhibit a higher concentration of H2O2-induced PDGFR signaling, which is known to act through an oxidative inhibition of protein tyrosine phosphatase. Finally, we showed that lutein functionally inhibited PDGF-induced VSMC migration, whereas its stereo-isomer zeaxanthin did not, revealing a special action of lutein on VSMCs.
It has been reported that higher quantities of dietary lutein were associated with lower risks of total stroke in the Health Professionals' Follow-Up Study. Moreover, two other key studies have provided support for a role of lutein and zeaxanthin in prevention of cardiovascular diseases, which shows inverse correlation of plasma lutein concentration and carotid intima-media thickness. In an in-vitro study, lutein and other carotenoids such as lycopene have been shown to reduce adhesion molecules expression in human aortic endothelial cells. This reflects a possible role of lutein in the prevention of atherosclerosis. However, dietary lutein stimulated delayed type hypersensitivity response, the number of CD4+ Th cells, and IgG production in dogs, suggesting its presence in peripheral areas and a possible protective role of lutein in vascular system.
Antimutagenic activity of natural xanthophylls against aflatoxin B1 in Salmonella typhimurium.
 Abstract:
Abstract:
Carotenoids (carotenes and xanthophylls) are excellent antioxidants with antimutagenic and anticarcinogenic properties. They occur naturally in some foods such as carrots, red tomatoes, butter, cheese, paprika, palm oil, corn kernels, Marigold petals, annatto, and red salmon. In the present study, we used the Salmonella plate incorporation test to examine the effect of xanthophylls extracted from Aztec Marigold (Tagetes erecta) on the AFB1 mutagenicity, using tester strain YG1024. The effect of lutein on the DNA-repair system in YG1024 was investigated by a pre-incubation test. In a dose-response curve of AFB1, the mutagenic potency was 1,031 revertants/nmol. The dose of 0.5 microgram AFB1/ plate was chosen for the antimutagenicity studies. Pure lutein and xanthophylls from Aztec Marigold flower (oleoresin and xanthophyll plus) inhibited the mutagenicity of AFB1 in a dose-dependent manner. The pigments were more efficient at inhibiting the AFB1 mutagenicity than pure lutein. The percentages of inhibition on AFB1 mutagenicity were 37, 66, and 76% for lutein, oleoresin, and xanthophyll plus at the dose of 2 micrograms/plate, respectively. Lutein had a modest effect on the DNA-repair system of YG1024. In spectrophotometric studies, a new absorption peak was detected at 378 nm when lutein and AFB1 were incubated together, and lutein reacted with AFB1 metabolites. The results suggest that the inhibitory mechanism of lutein against AFB1 mutagenicity is most probably the result of a combination of the following events: formation of a complex between lutein and AFB1, direct interaction between lutein and AFB1 metabolites, and finally that the lutein may also affect the metabolic activation of AFB1 by S9 and the expression of AFB1-modified Salmonella DNA.