Definition

Ebola virus disease (EVD) or Ebola hemorrhagic fever (EHF) is a severe, often-fatal disease in humans and nonhuman primates (monkeys, gorillas and chimpanzees) that has appeared sporadically since its initial recognition in 1976.
The disease is caused by infection with Ebola virus, named after a river in the Democratic Republic of the Congo in Africa, where it was first recognized. The virus is one of two members of a family of RNA viruses called the Filoviridae.
There are five identified subtypes of Ebola virus. Four of the five have caused disease in humans: Ebola-Zaire , Ebola-Sudan , Ebola-Ivory Coast and Ebola-Bundibugyo .
The fifth, Ebola-Reston, has caused disease in nonhuman primates, but not in humans.
| Species name | Virus name (abbreviation) |
| Bundibugyo ebolavirus | Bundibugyo virus (BDBV) |
| Sudan ebolavirus | Sudan virus (SUDV) |
| Taï Forest ebolavirus | Taï Forest virus (CIEBOV) |
| Zaire ebolavirus | Zaire virus (ZEBOV) |
Epidemiology
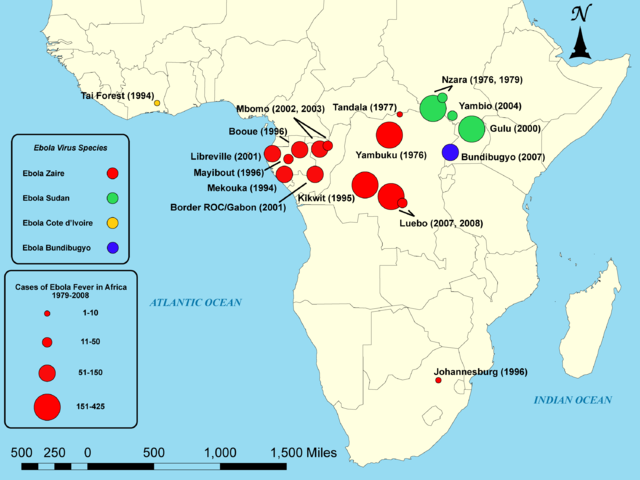
Outbreaks of EVD have mainly been restricted to Africa.
The origin in nature and the natural history of Ebola virus remain a mystery. It appears
that the viruses are zoonotic, i.e. that they are transmitted to humans from discrete life cylces in animals or insects. All attempts to trace the virus source from outbreak index cases, however, have failed to uncover a reservoir. Whatever the original source, person-to-person transmission is the means by which outbreaks and epidemics progress. This involves direct contact with infected blood, secretions, organs or semen. Hospital-acquired infections have been frequent, and many health care workers have been infected while attending patients. Transmission also occurs through preparation of the dead for burial. In the 1976 Zairian Ebola epidemic, many cases could be linked to the use of contaminated syringes and needles. In all episodes, isolation of patients and the use of barrier nursing procedures, including the use of protective clothing and respirators, have been sufficient to interrupt transmission. Although aerosol transmission has not been implicated in outbreaks to date, it cannot be totally discounted. Also if no non-human primates have been implicated in known outbreaks of Ebola haemorrhagic fever in humans, they are usceptible to infection. There is, however, no indication of where the infection originated. The obvious susceptibility of the chimpanzees makes them unlikely candidates for natural reservoirs of the virus.
(CDC Ebola Virus)
Patogenesis
Although its clinical course is well known, the specific mechanisms underlying the pathogenicity of Ebola virus have not been clearly delineated. This is due, in part, to the difficulty in obtaining samples and studying the disease in the relatively remote areas in which the outbreaks occur. In addition, a high degree of biohazard containment is required for laboratory studies and clinical analysis. Isolation of the viral cDNAs and the development of expression systems have allowed the study of Ebola virus gene products under less restrictive conditions and facilitated an understanding of the mechanisms underlying virally induced cell damage.
(Ncbi)
Risk factors
Plants, arthropods, and birds are considered as possible reservoirs; however, bats are considered the most likely candidate.Of 24 plant species and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected. The absence of clinical signs in these bats is characteristic of a reservoir species.
Bats drop partially eaten fruits and pulp, then terrestrial mammals such as gorillas and duikers feed on these fallen fruits. This chain of events forms a possible indirect means of transmission from the natural host to animal populations, which have led to research towards viral shedding in the saliva of bats. Fruit production, animal behavior, and other factors vary at different times and places which may trigger outbreaks among animal populations. Transmission between natural reservoirs and humans are rare, and outbreaks are usually traceable to a single index case where an individual has handled the carcass of gorilla, chimpanzee, or duiker. The virus then spreads person-to-person, especially within families, hospitals, and during some mortuary rituals where contact among individuals becomes more likely.The virus has been confirmed to be transmitted through body fluids. Transmission through oral exposure and through conjunctiva exposure is likely and has been confirmed in non-human primates.
Symptoms
The mean incubation period, best calculated currently for EVD outbreaks due to EBOV infection, is 12.7 days, but can be as long as 25 days. EVD begins with a sudden onset of an influenza-like stage characterized by general malaise, fever with chills, arthralgia and myalgia, and chest pain. Nausea is accompanied by abdominal pain, anorexia, diarrhea, and vomiting.
Respiratory tract involvement is characterized by
• pharyngitis with sore throat
• cough
• dyspnea
• hiccups
The central nervous system is affected as judged by the development of
• severe headaches
• agitation and confusion
• fatigue
• depression
• seizures
• sometimes coma
Cutaneous presentation may include
• maculopapular rash
• petechiae
• purpura
• ecchymoses
• hematomas (especially around needle injection sites).
Development of hemorrhagic symptoms is generally indicative of a negative prognosis. However, contrary to popular belief, hemorrhage does not lead to hypovolemia and is not the cause of death (total blood loss is low except during labor). Instead, death occurs due to multiple organ dysfunction syndrome (MODS) due to fluid redistribution, hypotension, disseminated intravascular coagulation, and focal tissue necroses.

Hemorrhage
All patients show some extent of coagulopathy and impaired circulatory system symptomology. Bleeding from mucous membranes and puncture sites is reported in 40–50% of cases, while maculopapular rashes are evident in approximately 50% of cases. Sources of bleeds include hematemesis, hemoptysis, melena, and aforementioned bleeding from mucous membranes (gastroinestinal tract, nose, vagina and gingiva). Diffuse bleeding, however, is rare, and is usually exclusive to the gastrointestinal tract.
Virology
Genome :ebolavirions contain linear nonsegmented, single-stranded, non-infectious RNA genomes of negative polarity. Ebolavirus genomes are approximately 19 kilobase pairs long and contain seven genes. The genomes of the five different ebolaviruses (BDBV, ZEBOV, RESTV, SUDV, and CIEBOV) differ in sequence and the number and location of gene overlaps.
Structure :Ebolavirions consist of seven structural proteins. At the center is the helical ribonucleocapsid, which consists of the genomic RNA wrapped around a polymer of nucleoproteins . Associated with the ribonucleoprotein is the RNA-dependent RNA polymerase with the polymerase cofactor and a transcription activator . The ribonucleoprotein is embedded in a matrix, formed by the major and minor matrix proteins. These particles are surrounded by a lipid membrane derived from the host cell membrane. The membrane anchors a glycoprotein that projects 7 to 10 nm spikes away from its surface. While nearly identical to marburgvirions in structure, ebolavirions are antigenically distinct.
Replication :The ebolavirus life cycle begins with virion attachment to specific cell-surface receptors, followed by fusion of the virion envelope with cellular membranes and the concomitant release of the virus nucleocapsid into the cytosol. The viral RNA polymerase, transcribes the genes into positive-stranded mRNAs, which are then translated into structural and nonstructural proteins. Newly synthesized structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane. Virions bud off from the cell, gaining their envelopes from the cellular membrane they bud from. The mature progeny particles then infect other cells to repeat the cycle.
Pathophysiology
Endothelial cells, mononuclear phagocytes, and hepatocytes are the main targets of infection. After infection, in a secreted glycoprotein (sGP) the Ebola virus glycoprotein (GP) is synthesized. Ebola replication overwhelms protein synthesis of infected cells and host immune defenses. The GP forms a trimeric complex, which binds the virus to the endothelial cells lining the interior surface of blood vessels. The sGP forms a dimeric protein which interferes with the signaling of neutrophils, a type of white blood cell, which allows the virus to evade the immune system by inhibiting early steps of neutrophil activation. These white blood cells also serve as carriers to transport the virus throughout the entire body to places such as the lymph nodes, liver, lungs, and spleen. The presence of viral particles and cell damage resulting from budding causes the release of cytokines (specifically TNF-α, IL-6, IL-8, etc.), which are the signaling molecules for fever and inflammation. The cytopathic effect, from infection in the endothelial cells, results in a loss of vascular integrity. This loss in vascular integrity is furthered with synthesis of GP, which reduces specific integrins responsible for cell adhesion to the inter-cellular structure, and damage to the liver, which leads to coagulopathy.
(Pathogenesis of the Viral Hemorrhagic Fevers,2012 , Medscape)

Diagnosis
EVD is clinically indistinguishable from Marburg virus disease (MVD), and it can also easily be confused with many other diseases prevalent in Equatorial Africa, such as other viral hemorrhagic fevers, falciparum malaria, typhoid fever, shigellosis, rickettsial diseases such as typhus, cholera, gram-negative septicemia, borreliosis such as relapsing fever or EHEC enteritis.
The most important indicator that may lead to the suspicion of EVD at clinical examination is the medical history of the patient, in particular the travel and occupational history and the patient's exposure to wildlife. EVD can be confirmed by isolation of ebolaviruses from or by detection of ebolavirus antigen or genomic or subgenomic RNAs in patient blood or serum samples during the acute phase of EVD. Filovirions can easily be visualized and identified in cell culture by electron microscopy due to their unique filamentous shapes, but electron microscopy cannot differentiate the various filoviruses alone despite some overall length differences. Immunofluorescence assays are used to confirm ebolavirus presence in cell cultures.
Prevention
Ebola prevention in Africa presents many challenges. Because the identity and location of the animal host of Ebola virus are unknown, there are few established primary Ebola prevention measures.
If cases of Ebola do appear, current social and economic conditions often favor the spread of an epidemic within healthcare facilities; therefore, healthcare providers must be able to recognize a case of Ebola should one appear. They must also have the capability to perform Ebola diagnostic tests and be ready to employ practical Ebola isolation precautions or barrier nursing techniques. These techniques include:
* The use of infection-control measures, including complete sterilization of equipment
* The isolation of patients with Ebola hemorrhagic fever from contact with unprotected people
* The wearing of protective clothing, such as masks, gloves, gowns, and goggles
The aim of all of these techniques is to avoid any person’s contact with the blood or secretions of any patient, including those who are deceased.
(http://ebola.emedtv.com/ebola/ebola-prevention.html)

Currently, there are no vaccines or effective therapies available for human use. Progress in understanding the geneses of the pathophysiological changes that make filoviral infections of humans so destructive has been slow, primarily because these viruses require special containment for safe research. However, an increasing understanding of the molecular mechanisms of filoviral pathogenesis, facilitated by the development of new tools to elucidate critical regulatory elements in the viral life cycle, is providing new targets that can be exploited for therapeutic interventions. In addition, substantial progress has been made in developing recombinant vaccines against these viruses.
(Ebola and Marburg viruses: pathogenesis and development of countermeasures,2005)
Francesco Rivarossa
Elena Lamprillo