INTRODUCTION
Hypokalemic periodic paralysis (hypoPP) is the most common form of periodic paralyses in man, althought it is still a rare disease, with a prevalence about 1 in 100 000. The major symptoms of hypoPP are episodes of generalised paralysis of duration related to the gravity of the disease.
Mild hypokalemia is often without symptoms, although it may cause a small elevation of blood pressure,and can occasionally provoke cardiac arrhythmias.
Moderate hypokalemia, with serum potassium concentrations of 2.5–3 mEq/L (Normal Level: 3.5–5.0 mEq/L), may cause muscle weakness, myalgia, and muscle cramps, and constipation (from disturbed function of smooth muscle).
With more severe hypokalemia, flaccid paralysis and hyporeflexia may result. Respiratory depression from severe impairment of skeletal muscle function is found in many patients. Rabdomyolisis can occur with a profound hypokalemia. Paralytic attack occur after excessive ingestion of carbohydrates or several hours following strenous exercise, commonly starting in the second half of the night and symptoms relief only occurs in the late morning.
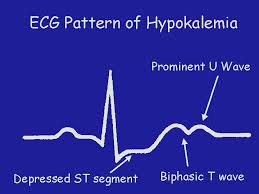
Some electrocardiographic (ECG) findings associated with hypokalemia include flattened or inverted T waves, a U wave and ST depression. Due to prolonged repolarization of ventricular Purkinje fibers, a prominent U wave occurs, that is frequently superimposed upon the T wave and therefore produces the appearance of a prolonged QT interval.
CAUSE OF HYPOKALIEMIC PERIODIC PARALYSIS IN OUR PATIENTS
Previous genetic studies reveled mutations in the L-type voltage-gated calcium channel in the CACNA1S gene, associated with hypokalemic periodic paralysis. In addition, several mutation (Arg699His, Arg672His, ArgGly and Arg672Ser) in the voltage sensor of the skeletal muscle sodium channel have been found in families with hypoPP.
During the skeletal muscle contraction the action potential propagates by activating voltage-gated sodium channels along the axon toward the neuromuscular junction. When it reaches the junction, it causes a calcium ion influx through voltage-gated calcium channels. The depolarization activates L-type voltage-dependent calcium channels in the T tubule membrane in the adjacent sarcoplasmatic reticulum.
Activated voltage-gated calcium channels physically interact with calcium-release channels to activate them, causing the sarcoplasmatic reticulum to release calcium. In the first mutation (CACNA1S - L-type voltage-dependent calcium channels) the calcium can’t enter flowing from the sarcoplasmatic reticulum to the intracellular environment and the contraction of the muscle is blocked. This may be the reason of the paralysis. Despite the knowledge of the hypoPP-causing mutations, the pathophisiology of the disease remains unclear.
Voltage-gated sodium channels are responsible for the initiation and propagation of action potential excitable cells. To regulate the action potential properly, the depolarizing sodium current needs to be quickly activated and inactivated. If inactivation in too slow or incomplete, as in the case of our second mutation (Arg699His, Arg672His, ArgGly and Arg672Ser), the repolarization phase of the action potential is delayed and a stable resting potential cannot be maintained: the sodium keep on entering inside the cell in a low quantity if the inactivation is incomplete or in a higher quantity in a slow inactivation, until the channel is inactivated.
In both cases there is an extra quantity of sodium inside the cell that must be expelled with the Na+/K+ pump. The obvious effect is an extra quantity of potassium inside that enters through the pump related to an exit of sodium. The potassium is concentrated inside the cells and the percentage in the blood is really low. This may reduce the number of functional sodium channel at resting membrane potential and contribute to the long-lasting periods of paralysis experienced by hypoPP patients.
TREATMENTS
- General (as in our patients)
Drugs administered have the task to solve the lack of potassium, not directly to decrease the loss. There are some commercially available drugs that block the transport of glucose, decreasing the potassium loss caused by carbohydrate metabolism, but they have hypokalaemia as a contraindication. This is the reason why they are not administered.
The most common are:
- KCl-retard: extended-release tablet, is a supplement to the lack of potassium.
- Concentrate for solution for infusion: 10 ml in each vial. In cases of hypokalaemia the vials may not be diluted. The amount of potassium taken from the vials in a hypokalaemic, leads to death a healthy person (intravenous potassium is one of the punishments of death in the USA) while lower doses can give vomiting and diarrhea.
The dosage varies from patient to patient, both as a quantity, as timing. Patients with mild hypokalemia may take only retardant potassium, extended-release tablets.
In the histidine mutants, in case of acidosis, the reduction of pH to 6.9 obtains a therapeutic effect activating the H+/K+ pump which increases the ematic potassium concentration while reducing the ematic pH
OTHER GENERAL CAUSES
- Inadequate potassium intake
Perhaps the most obvious cause is insufficient consumption of potassium (that is, a low-potassium diet) or starvation. However, without excessive potassium loss from the body, this is a rare cause of hypokalemia.
The loss of potassium is often associated with heavy fluid losses that "flush" potassium out of the body. This is a consequence of diarrhea, excessive perspiration, or losses associated with surgical procedures. Vomiting can also cause hypokalemia, although not much potassium is lost from the vomitus.
- Certain medications can cause excess potassium loss in the urine. Diuretics, including thiazide diuretics are a common cause of hypokalemia. Other medications such as the antifungal, amphotericin B, or the cancer drug, cisplatin, can also cause long-term hypokalemia.
- A special case of potassium loss occurs with diabetic ketoacidosis. Hypokalemia is observed with low total body potassium and a low intracellular concentrations of potassium. In addition to urinary losses from polyuria and volume contraction, there is also obligate loss of potassium from kidney tubules as a cationic partner to the negatively charged ketone, β-hydroxybutyrate.
- Hypomagnesemia can cause hypokalemia. Magnesium is required for adequate processing of potassium. This may become evident when hypokalemia persists despite potassium supplementation.
- Alkalosis can cause transient hypokalemia by the shift of potassium from the plasma and interstitial fluids into cells; perhaps mediated by stimulation of Na+/H+ exchange and a subsequent activation of Na+/K+ pump activity. Or if there is an acute rise of plasma HCO3- concentration, the proximal tubule to reabsorb this anion, will excret potassium as an obligate cation partner to the bicarbonate.
- Potassium is also lost via aldosterone-mediated mechanisms. Disease states that can cause hypertension and excessive urinary losses of potassium. These include renal artery stenosis and tumors, Conn's syndrome (primary hyperaldosteronism). Cushing's syndrome can also lead to hypokalemia due to excess cortisol binding the Na+/K+ pump and acting like aldosterone. Wikipedia
CLINICAL CASES
We have directly carried out studies about two brothers suffering from the disease.
Patient n°1 : Matteo Taricco
Age: 20
First manifestation of the disease: 14 years, due to an intense effort during a football match.
Family history: the mother is a carrier of the mutation, there were no other signs of the mutation in the maternal grandparents (the disease can skip generations).
Data collected from the interview of the patient:
Metteo Taricco has a mutation that causes periodic paralysis due to declines in the potassium level in the blood. He is the second son and he had facilities than Mr. Taricco Giovanni, his brother, so at the first paralysis the disease was immediately diagnosed. Seemingly he has a normal life but he has to follow a certain protocol in order to carry out normal life activities.
Diet: inability to eat carbohydrates in the afternoon and at night (pasta, bread, cakes) or in small quantity during the day. Some elements are more influential than others, and the reason is still unknown: the pizza is an example, which involves paralysis the following day. The patient can’t drink alcool and he has to drink high amounts of water, above the average of a normal person not suffering from the disease; otherwise the urine acquire a very strong odor and a unnaturally yellowish coloration. The patient has problems in managing his diet, but a correct alimentation is not a sufficient reason to avoid hypokalaemic crises: even stress, physical activities, fear and surgical operations are influential.
Clinical manifestations: the drop consists of a reduction of potassium which paralyzes the muscles . It mainly occurs in the night due to a lack of mobility of the limbs. The paralysis can be total or partial depending on the moment or on the gravity of the patient. Mr.Taricco, in his individual case, in average has a total paralysis every two weeks, that it could be exceeded with a different number of hours: in some cases in 4-6 hours, in other cases in 15-20 hours. He has often difficulties upon waking in the control of the hands and arms, while the lower part of the body is fully functional. This points out that potassium may only affect certain areas of the body and leave other fully active. In his particular case eyelids and mouth are never involved , and he tells us about other cases in which there is also the paralysis of the previous two organs, or in others where the declines are much milder . The excessive loss of potassium does not end in the course of life but usually has a slight improvement. Even if in the patient is not clear yet, according to the predictions and previous cases he can reach a more stable situations at the age of 40 (the brother is 3 years older and he takes fewer vials) .
Treatments: the patient takes 6 daily vials and sometimes KCl-retard tablets with a retardant effect. The quantity of potassium taken by Mr.T increases in time of stress (ex: exams, sports competitions), changing in the temperature, before and after great physical efforts. In such cases it may reach up to 8 vials per day. It 's a kind of self-management because no one doctor can prescribe an exact quantity of potassium to take. Not being able to have under daily examination blood potassium levels, by the time he learned to predict some of the possible unwell. He does not take medicines to prevent the hypokalemic crisis, because there aren’t yet pharmaceutical substances able to prevent the loss.
Patient N° 2: Giovanni Taricco
Age: 23
First manifestation of the disease: 14 years caused by stress after the exam in middle school.
Family history: the mother is a carrier of the mutation, there were no other signs of the mutation in the maternal grandparents (the disease can skip generations)
Data collected from the interview of the patient (diet and manifestation check Mr. Matteo Taricco)
Treatments: the patient takes about 2-3 vials per day with, sometimes some tablets of KCl-retard. His crisis are less frequent, as the need for a continuous supply of potassium.