DEFINITION
Infant respiratory distress syndrome ,also called Neonatal RDS, is a syndrome caused by developmental insufficiency of pulmonary surfactant commonly suffered by premature babies born before 28–32 weeks of gestation. It can also result from a genetic problem with the production of surfactant associated proteins and it is a major cause of morbidity and mortality in preterm infants.
(MeSH)
EPIDEMIOLOGY
RDS is the most common respiratory disorder of premature newborns and its incidence is directly proportional to the degree of prematurity.

Figure 1. Incidence of RDS by gestational period (A) and birth weight (B)
International occurence: Respiratory distress syndrome is encountered less frequently in developing countries than elsewhere, primarily because most premature infants who are small for their gestation are stressed in utero because of malnutrition or pregnancy-induced hypertension. In addition, because most deliveries in developing countries occur at home, accurate records in these regions are unavailable to determine the frequency of respiratory distress syndrome.
In the United States, respiratory distress syndrome has been estimated to occur in 20,000-30,000 newborn infants each year and is a complication in about 1% pregnancies. Approximately 50% of the neonates born at 26-28 weeks' gestation develop respiratory distress syndrome, whereas less than 30% of premature neonates born at 30-31 weeks' gestation develop the condition.
Generally RDS occurs most often in white premature infants.
(Emedicine RDS)
SYMPTOMS
Most of the time symptoms begins shortly after birth and they manifest by dyspnea, cyanosis,unusual breathing movements and other signs that are the most evident. However, they may not be seen for several hours. As the disease progresses, the baby may develop ventilatory failure (rising carbon dioxide concentrations in the blood), and prolonged cessations of breathing ("apnea"). During the first day the patient requires more support,then the baby may be remarkably stable on adequate support.
(Medline)
(Wikipedia)
DIAGNOSIS
RDS is usually diagnosed by a combination of assessments, including the following:
- Appearance, color, and breathing efforts (indicate a baby's need for oxygen).
- Chest X-rays of lungs.
- Blood gases (tests for oxygen, carbon dioxide and acid in arterial blood). These often show low oxygen and excess acid in the body fluids.
- Echocardiography,used to rule out heart problems that might cause symptoms similar to RDS.
- Lab tests, used to rule out infection as a cause of breathing problems.
(Stanford Children's Health)
PATHOGENESIS

Figure 2. Pathogenesis of Infant RDS
The transition from fetus to infant involves many complex adaptations at birth; the most important is the function of the lungs as a gas exchange organ. Preterm surfactant-deficient infants are less well equipped to deal with this transition, because surfactant helps prevent collapse of the terminal air-spaces. Optimum gas exchange is achieved through matching of ventilation and perfusion. A surfactant deficient lung is characterized by collapsed air-spaces alternating with hyper-expanded areas, vascular congestion and in time, hyaline membranes. This blocks gas exchange and as a result blood oxygen levels fall and carbon dioxide rises, resulting in rising blood acid levels (that cause pulmonary vasoconstriction, resulting in impaired endothelial and epithelial integrity with leakage of proteinaceous exudate) and hypoxia. Structural immaturity, as manifest by decreased number of gas-exchange units and thicker walls, also contributes to the disease process.
The relative deficiency of surfactant decreases lung compliance (Figure 1) and functional residual capacity, with increased dead space. The resulting large V/Q mismatch and right-to-left shunt may involve as much as 80% of the cardiac output.
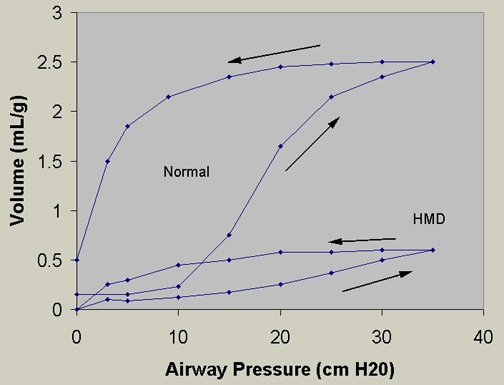
Figure 3. Pressure-volume curves. Infant RDS lungs (or HMD, hyaline membrane disease) require a higher pressure to reach a certain volume compared with normal lungs
(Pathophysiology of neonatal respiratory distress syndrome,PubMed)
(Wikipedia, IRDS)
RISK FACTORS
The greatest risk factor for respiratory distress syndrome is prematurity, even if the syndrome does not occur in all premature newborns. Although most babies with
RDS are premature, other factors can influence the chances of developing the disease. These include the following:
- White or male babies
- Previous birth of baby with RDS
- Cesarean delivery
- Perinatal asphyxia
- Cold stress (a condition that suppresses surfactant production)
- Perinatal infection
- Multiple births (multiple birth babies are often premature)
- Infants of diabetic mothers (too much insulin in a baby's system due to maternal diabetes can delay surfactant production)
- Babies with patent ductus arteriosus
Infants born to mothers with glucose intolerance (diabetic) are at an increased risk of morbidity and mortality related to respiratory distress (Infant of diabetic mothers).
It was found an association between maternal hypothyroidism and the risk of RDS in newborns.
Thyroid hormones are very important for the development of the fetus, especially of his nervous system and also for the development of lung surfactant (formerly known fundamental role in the adults), in fact, it is now clear the demonstration of the transfer of maternal thyroid hormones across the placenta.
Studies have been conducted that have shown that newborns of hypothyroid mothers need of intensive care after birth, especially due to complications such as premature birth, low birth weight and increased neonatal respiratory distress (Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease,2013)
(Fetal and neoantal aspects of maternal hypothyroidism,2012)
Moreover, associations with genetic disorders have been noted. The available studies mainly concentrate on surfactant proteins (SP-A, SP-B); mutations in the genes encoding the surfactant proteins B and C and the phospholipid transporter are associated with respiratory distress and interstitial lung disease in the pediatric population.The most common condition found are the Hereditary SP-B deficiency (an autosomal recessive disorder with mutations required on both alleles in order to cause disease) and mutations in the ABCA3 gene that lead to a surfactant metabolism dysfunction. (Genetic Disorders of Surfactant Dysfunction,2010)
The candidate-gene approach was used to study the association between the surfactant protein B (SP-B) mRNA expression and RDS (Correlation between surfactant protein B mRNA expression and neonatal respiratory distress syndrome,2012)

Figure 4. Human surfactant protein B (SP-B) gene

Figure 5. Human ABCA3 gene and protein illustrating the location of mutations found throughout the gene and protein
COMPLICATIONS
Children suffering from RDS can sometimes develop complications or problems as side effects of treatment.
Some complications associated with RDS include pneumomediastinum , pneumothorax ,
pneumopericardium and pulmonary interstitial emphysema , these are all linked to air leak. Moreover, infections may complicate the management of respiratory distress syndrome and may manifest in various ways as a result of the use of respiratory equipment or surfactant therapy.
Patent ductus arteriosus with increasing left-to-right shunt is a complication that lead to an increse of blood pressure in the lung arteries.
As a result of chronic lung disease, the worst complication may be bronchopulmonary dysplasia , that develops when infant's lungs becoming irritated or inflamed, as a consequence of the use of ventilator support in RDS therapy. Morphological changes in preterm infants with bronchopulmonary dysplasia have functional consequences on lung volume, ventilation inhomogeneity and respiratory mechanics. Some studies have shown lower lung volumes and increased ventilation inhomogeneity in BPD infants.

Figure 6. Box plots of the respective lung function values by subject groups.Subjects were grouped according to post-conceptional age at birth (term-born and preterm) and according to disease state based on ATS definition of BPD (healthy preterm, mild, moderate and severe BPD)
(Prognosis of RDS)
THERAPY
Treatment for respiratory distress syndrome usually begins as soon as an infant is born, sometimes in the delivery room.Most infants who show signs of
RDS are quickly moved to a neonatal intensive care unit. The most important treatments for
RDS are:
- Surfactant replacement therapy , where the surfactant usually is given through a breathing tube that allows it to go directly into the baby's lungs.
- Breathing support from a ventilator or nasal continuous positive airway pressure (NCPAP) machine. About this treatment, trials have been conducted to see if it was possible to reduce the risk of bronchopulmonary dysplasia, consequence of invasive ventilation. The results show the benefit of nasal intermittent positive air pressure (NIPPV) in preterm infants with RDS, with a significant reduction in the need for intubation and invasive mechanical ventilation within the first 72 hours of life (Nasal Intermittent Positive-Pressure Ventilation vs Nasal Continuous Positive Airway Pressure for Preterm Infants With Respiratory Distress Syndrome,2012)
- Oxygen therapy . This treatment ensures that the infants' organs get enough oxygen to work well.
Other treatments for RDS include medicines, especially antibiotics, to control infections and supportive therapy that include using a radiant warmer or incubator to keep infants warm,Giving fluids and nutrients through needles inserted into the infants' veins and continuous monitoring of physiological parameters.
Figure 7. Pneumonitor Device
(RDS treatment, NIH)
PREVENTION
In pregnancies of greater than 30 weeks, the fetal lung maturity may be tested by sampling the amount of surfactant in the amniotic fluid by amniocentesis, wherein a needle is inserted through the mother's abdomen and uterus. Several tests are available that correlate with the production of surfactant. These include the lecithin-sphingomyelin ratio ("L/S ratio"), the presence of phosphatidylglycerol (PG), and more recently, the surfactant/albumin (S/A) ratio. For the L/S ratio, if the result is less than 2:1, the fetal lungs may be surfactant deficient. The presence of PG usually indicates fetal lung maturity. For the S/A ratio, the result is given as mg of surfactant per gm of protein. An S/A ratio <35 indicates immature lungs, between 35-55 is indeterminate, and >55 indicates mature surfactant production.
Most cases of infant respiratory distress syndrome can be prevented if mothers who are about to deliver prematurely can be given glucocorticoids, one group of hormones. This speeds the production of surfactant. For very premature deliveries, a glucocorticoid is given without testing the fetal lung maturity. The major organizations have recommended antenatal glucocorticoid treatment for women at risk for preterm delivery prior to 34 weeks of gestation. antenatal glucocrticoids accelerate lung development, aspecially the production of surfactant (Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth,2006)