DEFINITION
A short protein description with the molecular wheight, isoforms, etc...
Use, when available, the link to Wikipedia (Es Trypsin)
External links not available on Wikipedia have to be added here
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage


PTN and MK have high lysine; that could correspond to low histones
h3. SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,
biological function
Ligands
In mammals PTN (pleiotrophin) also known as HB-GAM (heparin-binding growth-associated molecule) [39], OSF-1 (osteoblast-specific factor-1) [40], HARP (heparin affinity regulatory peptide) [41] and HBNF (heparin-binding neurotrophic factor) [42]; and MK (midkine) [43], also known as RIHB (retinoic acid-inducible heparin-binding protein) [44], have been postulated to be the activating ligands for ALK [6,45]. MK and PTN are small, heparin-binding growth factors implicated in diverse processes such as neural development, cell migration and angiogenesis [46,47]. The observation that PTN could function as a ligand for ALK arises from the isolation of a small portion of the extracellular region of ALK which was identified upon screening a human foetal brain phage display cDNA library for PTN-binding partners [6]. Subsequently, the PTN-related protein MK was identified as an ALK ligand. Furthermore, antibodies directed toward the ALK extracellular domain could inhibit the in vitro ligand–receptor interaction, suggesting that MK and PTN bind ALK
Anaplastic lymphoma kinase: signalling in development and disease 2009
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC USE

The anaplastic lymphoma kinase in the pathogenesis of cancer 2008
more links
Pathobiology of ALK+ anaplastic large-cell lymphoma 2007



Signalling via dALK occurs via binding of the ligand Jeb, downstream activation of ERK and transcription of downstream target genes. Signalling via mammalian ALK is thought to occur via ligand-mediated dimerization in response to the MK and PTN ligands. ALK mediates signalling via the JAK/STAT, RAS/MAPK, PI3K and PLCγ pathways. Activation of ALK via RPTPβ/ζ, independently of direct ALK–ligand interactions has also been proposed. Lastly, ALK is proposed to function as a dependency receptor which is cleaved by caspase 3 (Casp. 3) in the absence of ligand, thereby promoting apoptosis.

Coupling histone homeostasis to centromere integrity via the ubiquitin-proteasome system 2010
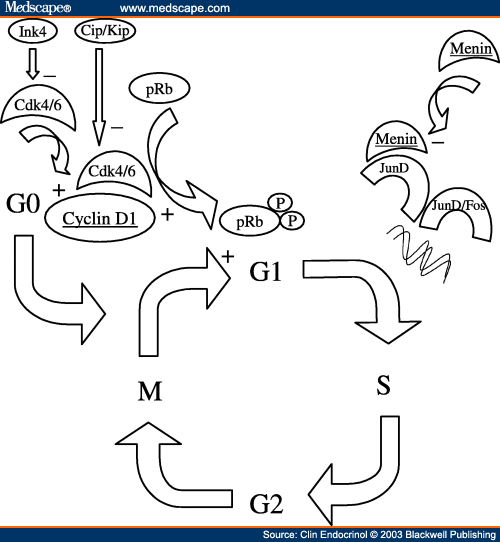
Suppression of lung adenocarcinoma through menin and polycomb gene-mediated repression of growth factor pleiotrophinMenin suppresses lung cancer through PTN 2009
ALK and its ligands bear different info about AA

while ALK and MEN1 (menin) are very similar.

and lack some AA necessary for cell cycle. For that reason MEN1 is an oncosuppressor.
Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. 2008
Menin, the product of the Men1 gene mutated in familial multiple endocrine neoplasia type 1 (MEN1), regulates transcription in differentiated cells. Menin associates with and modulates the histone methyltransferase activity of a nuclear protein complex to activate gene expression. However, menin-dependent histone methyltransferase activity in endocrine cells has not been demonstrated, and the mechanism of endocrine tumor suppression by menin remains unclear. Here, we show that menin-dependent histone methylation maintains the in vivo expression of cyclin-dependent kinase (CDK) inhibitors to prevent pancreatic islet tumors. In vivo expression of CDK inhibitors, including p27 and p18, and other cell cycle regulators is disrupted in mouse islet tumors lacking menin. Chromatin immunoprecipitation studies reveal that menin directly associates with regions of the p27 and p18 promoters and increases methylation of lysine 4 (Lys-4) in histone H3 associated with these promoters. Moreover, H3 Lys-4 methylation associated with p27 and p18 is reduced in islet tumors from Men1 mutant mice. Thus, H3 Lys-4 methylation is a crucial function of menin in islet tumor suppression. These studies suggest an epigenetic mechanism of tumor suppression: by promoting histone modifications, menin maintains transcription at multiple loci encoding cell cycle regulators essential for endocrine growth control.
Genetic and Clinical Aspects of Primary Hyperparathyroidism: Inactivation of the MEN1 Gene - A Putative Tumour Suppressor Gene