The precursors

There is a cross-regulation between AMP and GMP synthesis: ATP is required for GMP synthesis and vice versa
From Monucleotide to Dinucleotide


All the 3 enzymes lack TrP pointing to a function alternative to cell proliferation (???)
From the evolutionary point of view (Glu/Gln low to high): KGUA-->KAD1 (cytoplasm)-->KAD2 (mitochondria)
GMP central role

Predicting and characterizing selective multiple drug treatments for metabolic diseases and cancer, 2012



GTP roles
Mitochondrial GTP Links Nutrient Sensing to β Cell Health, Mitochondrial Morphology, and Insulin Secretion Independent of OxPhos, 2019

Highlights
•
Enhanced mitochondrial GTP (mtGTP) production amplifies insulin secretion
•
mtGTP regulates PEPCK-M and cytosolic Ca2+, not OxPhos, to trigger insulin secretion
•
Exuberant secretion and enhanced insulin biosynthesis can coexist without toxicity
•
mtGTP turnover enhances insulin content, granule docking, and mitochondrial mass
Summary
Mechanisms coordinating pancreatic β cell metabolism with insulin secretion are essential for glucose homeostasis. One key mechanism of β cell nutrient sensing uses the mitochondrial GTP (mtGTP) cycle. In this cycle, mtGTP synthesized by succinyl-CoA synthetase (SCS) is hydrolyzed via mitochondrial PEPCK (PEPCK-M) to make phosphoenolpyruvate, a high-energy metabolite that integrates TCA cycling and anaplerosis with glucose-stimulated insulin secretion (GSIS). Several strategies, including xenotopic overexpression of yeast mitochondrial GTP/GDP exchanger (GGC1) and human ATP and GTP-specific SCS isoforms, demonstrated the importance of the mtGTP cycle. These studies confirmed that mtGTP triggers and amplifies normal GSIS and rescues defects in GSIS both in vitro and in vivo. Increased mtGTP synthesis enhanced calcium oscillations during GSIS. mtGTP also augmented mitochondrial mass, increased insulin granule number, and membrane proximity without triggering de-differentiation or metabolic fragility. These data highlight the importance of the mtGTP signal in nutrient sensing, insulin secretion, mitochondrial maintenance, and β cell health.
cellular localization
Dynamic Compartmentalization of Purine Nucleotide Metabolic Enzymes at Leading Edge in Highly Motile Renal Cell Carcinoma, 2019
Abstract
Compartmentalization is vital for biological systems at multiple levels, including biochemical reactions in metabolism. Organelle-based compartments such as mitochondria and peroxisomes sequester the responsible enzymes and increase the efficiency of metabolism while simultaneously protecting the cell from dangerous intermediates, such as radical oxygen species. Recent studies show intracellular nucleotides, such as ATP and GTP, are heterogeneously distributed in cells with high concentrations at the lamellipodial and filopodial projections, or leading edge. However, the intracellular distribution of purine nucleotide enzymes remains unclear. Here, we report the enhanced localization of GTP-biosynthetic enzymes, including inosine monophosphate dehydrogenase (IMPDH isotype 1 and 2), GMP synthase (GMPS), guanylate kinase (GUK1) and nucleoside diphosphate kinase-A (NDPK-A) at the leading edge in renal cell carcinoma cells. They show significant co-localization at the membrane subdomain, and their co-localization pattern at the membrane is distinct from that of the cell body. While other purine nucleotide biosynthetic enzymes also show significant localization at the leading edge, their co-localization pattern with IMPDH is divergent. In contrast, a key glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), predominantly localized in the cytoplasm. Mechanistically, we found that plasma membrane localization of IMPDH isozymes requires active actin polymerization. Our results demonstrate the formation of a discrete metabolic compartment for localized purine biosynthesis at the leading edge, which may promote localized nucleotide metabolism for cell migration and metastasis in cancers.
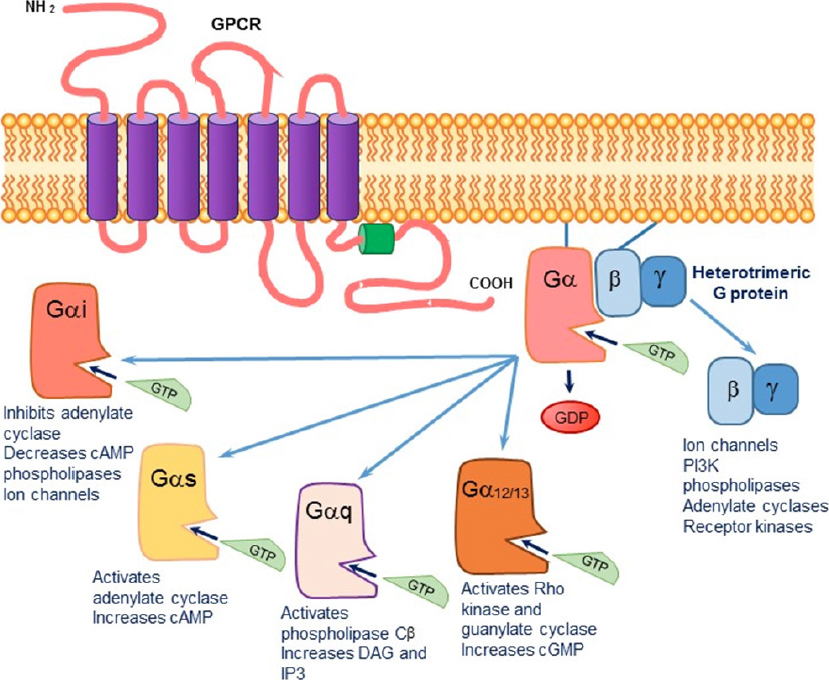
Figure 1. The signaling cascade initiated upon agonist binding to a G protein-coupled transmembrane receptor (GPCR). The exchange of GDP by GTP causes a dissociation of the Gα subunit from the βγ subunit. The activated alpha subunit can alter downstream effectors. Each of the alpha subunits has a specific set of second messengers, while the dissociated βγ subunit can independently activate a variety of effectors
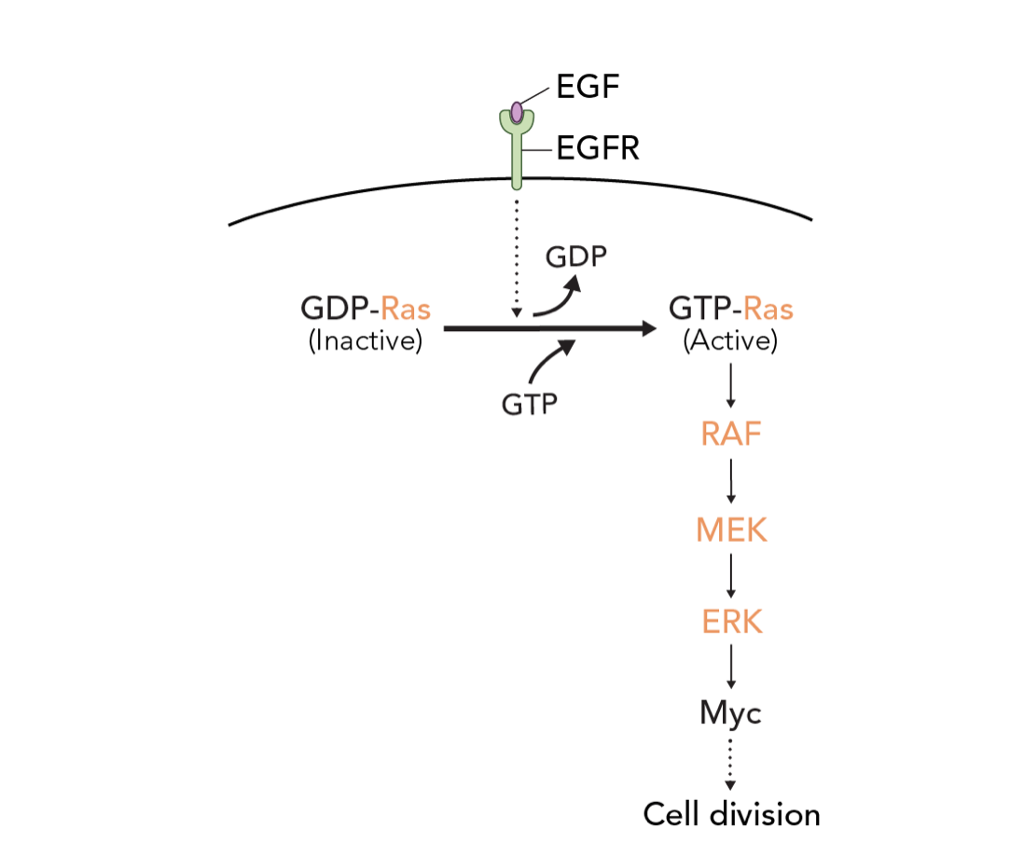
Activation of the cGMP/protein kinase G system in breast cancer by the dopamine receptor-1, 2019