CHOLESTEROL ABSORPTION.
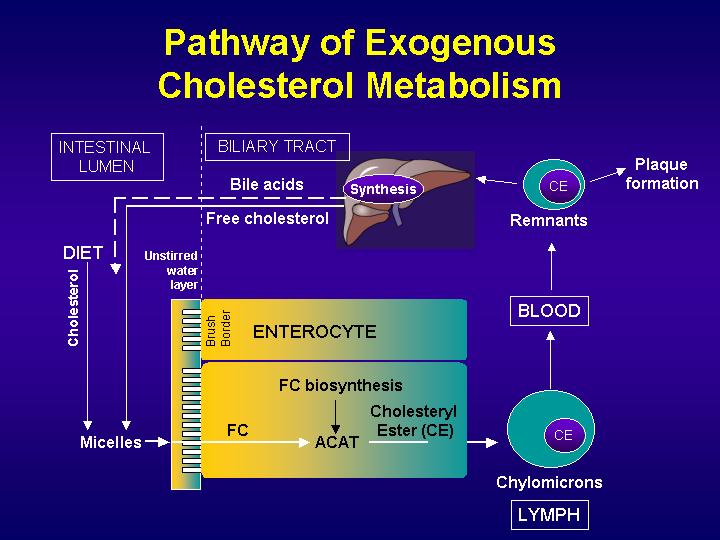
Fats in the diet consist of cholesterol as well as triglycerides. Dietary cholesterol is incorporated into micelles together with the bile cholesterol. Dietary triglycerides are partially broken down by pancreatic lipases into fatty acids and monoglycerides, which are also incorporated into micellar particles, made of bile salts and phospholipids, which emulsify fats.
Then, solubilized sterols enter the enterocytes of the duodenum and the jejunum either by passive diffusion or by a protein-mediated process. Cholesterol is esterified by acyl-CoA:cholesterol acyltransferase-2 (ACAT-2) to form cholesterol esters, which are secreted from the basolateral surfaces of the enterocytes as part of chylomicrons, which enters the lymphatic and then the general circulation.
BILE SALTS CIRCLE
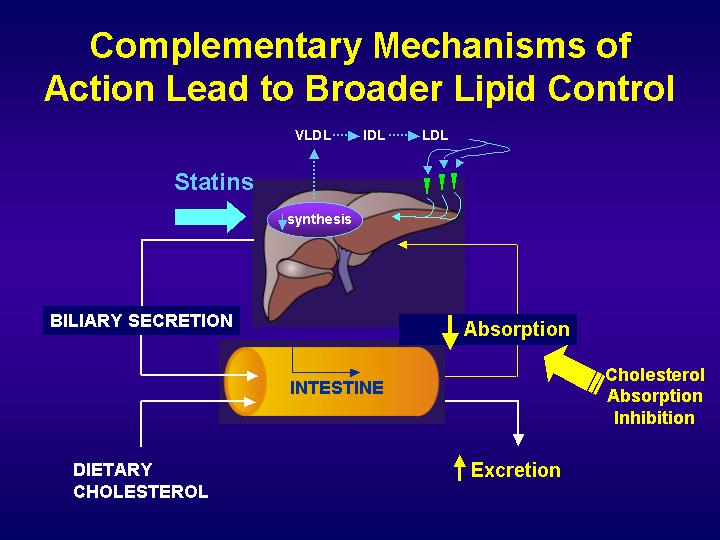
Bile salts are secreted at a rate of 24 g/day, but synthesized at a rate of only 0.4 g/day in the average individual. This is because, once bile salts have completed their functions in the biliary tree and intestine, almost all are reabsorbed in the distal ileum and returned to the liver through portal venous circulation. Less than 5% of bile salts are lost in the feces each day, which amounts to about 0.4 g/day. The synthesis of bile salts in the liver is adjusted by the body to match the fecal excretion. Considering that cholesterol is the substrate for bile salt synthesis in the liver, a loss of 0.4 g/day of bile salts translates to a loss of the same quantity of cholesterol (i.e., 0.4 g/day).
p<>. Cholesterol is secreted into bile at the rate of up to 2 g/day. The average diet consists of about 0.4 g/day of cholesterol. Therefore, the amount of cholesterol that is derived from bile in the intestine is up to 5-fold in excess of the amount that is taken in through the diet. The biliary cholesterol and dietary cholesterol are admixed in the intestine to form a pool of cholesterol molecules that are indistinguishable. The average individual absorbs 50% of the cholesterol that passes through the intestine each day. This means that 50% is lost in the feces, amounting to 1.2 g/day.
CHOLESTEROL TRANSPORTER
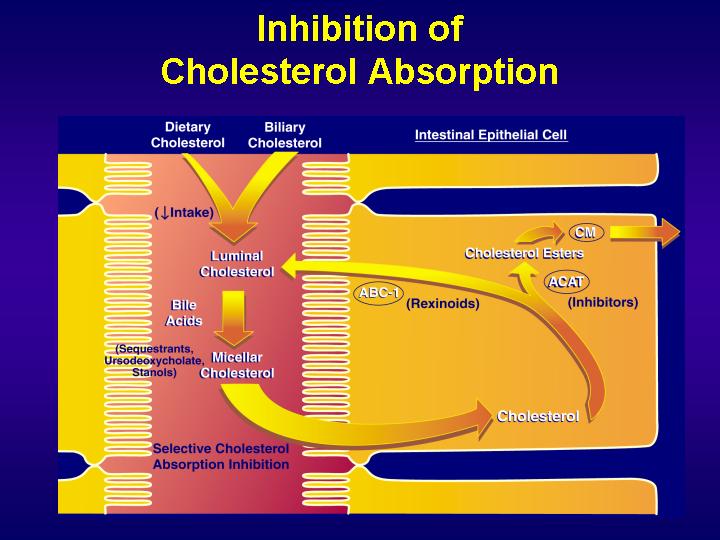
Mechanisms by which enterocytes absorb lipids is still controversial. In addition to a simple passive diffusion through the membrane, there is strong evidence for the existence of a protein-facilated mechanism. Different proteins have successively been implicated in intestinal lipid absorption.
Recently, a newly discovered protein, the Niemann-Pick C1 Like 1 (NPC1L1), has been shown to play a major role in cholesterol absorption since knock-out mice for this protein decreased intestinal cholesterol absorption by 80%.
ATP-binding cassette (ABC) transporters, ABCG5 and ABCG8, are supposed to limit sterol absorption and to promote bile sterol excretion in humans. ABCG5 and ABCG8 are members of the ATP-binding cassette transporter superfamily which contains membrane proteins that translocate a variety of substrates across extra- and intra-cellular membranes. Genetic variation in these genes in involved in a wide variety of human disorders, including cystic fibrosis, neurological disease, retinal degeneration, cholesterol and bile transport defects and drug resistance in tumour cells.
Conservation of the ATP-binding domains of these genes has allowed the identification and classification of new members of the superfamily based on nucleotide and protein sequence homology.
The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes
A typical ABC transporter has four domains or subunits, two of which are hydrophobic and are predicted to span the membrane multiple times in an alpha-helical conformation and two of which bind nucleotide (ATP) and are exposed to the cytoplasm
We can distinguish full-size transporters with two trans-membrane domains and two nucleotide-binding domains encoded by a single gene and half-size transporters with one nucleotide-binding domain and one trans-membrane domain, that either form homodimers or heterodimers. The trans-membrane domains provide the transport pathway for a particular substrate, and the nucleotide-binding domains power the transport by ATP hydrolysis.
The nucleotide-binding domains share considerable sequence homology across the entire family and have a similar three-dimensional fold that consists of a core nucleotide-binding subdomain that is common to other ATPases and an alpha-helical subdomain that is specific to ABC proteins. Conservation of the ATP-binding domains of these genes has allowed the identification and classification of new members of the superfamily based on nucleotide and protein sequence homology.
In a study in which transgenic mice with high-level expression of human ABCG5 and ABCG8, both on the apical membrane of enterocytes and on the canalicular membrane of hepatocytes, were generated, transgene over-expression only modestly affected plasma and liver cholesterol levels but profoundly altered cholesterol transport. The fractional absorption of dietary cholesterol was reduced by about 50%, and bile cholesterol levels were increased more than fivefold. No significant changes in the pool size, composition, and fecal excretion of bile acids were observed in the transgenic mice.. These results demonstrate that increased expression of ABCG5 and ABCG8 selectively drives bile neutral sterol secretion and reduces intestinal cholesterol absorption, leading to a selective increase in neutral sterol excretion and a compensatory increase in cholesterol endogenous synthesis.
Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol
The mechanism by which cholesterol is secreted in bile, could be explained with two hypotheses
The first one suggests that the ABCG5/ABCG8 heterodimer acts as a floppase, mediating the translocation of cholesterol from the inner to the outer leaflet of the canalicular membrane. After translocation of cholesterol, ABCG5/ABCG8 would then mediate the extrusion of cholesterol monomers from the extracellular leaflet of the canalicular membrane into the canalicular space, where mixed micelles and phospholipid vesicles act as acceptors emulsifying cholesterol monomers. The same mechanism could perhaps spiegare cholesterol extrusion from enterocytes into the intestinal lumen. The necessity of a floppase function can be questioned, as cholesterol has been shown to flip-flop between membrane leaflets spontaneously.
The second one suggests that the heterodimer is involved in decreasing the energy required to release cholesterol from the outer leaflet of the canalicular membrane. According to this proposal, ABCG5/ABCG8 would form a cholesterol binding complex “pushing” cholesterol partially into the lumen, using ATP hydrolysis as the driving force. Under these circumstances, the accessibility of cholesterol for extraction by bile salt/phospholipid micelles would be enhanced.
Scavenger receptor class B type I (SR-BI) is also a likely element of intestinal cholesterol absorption. SR-BI is a 82 kDa membrane protein mostly expressed in liver and steroidogenic tissues. It binds native high density lipoproteins (HDL), LDL or oxidized LDL and anionic phospholipids and mediates both the selective uptake of HDL cholesteryl ester by the liver and free cholesterol efflux from cells of peripheral tissues. SR-BI is also expressed in enterocyte brush border membranes mainly at the top of intestinal villosities and in the proximal part of intestine where cholesterol absorption mainly occurs. However, the importance of SR-BI in intestinal cholesterol absorption is not so sure since the disruption of SR-BI gene in mice does not affect cholesterol absorption. However, the process seems to be more complex and might depend on the combined actions of transporter proteins involved in uptake and efflux of cholesterol, which could compensate for the lack of SR-BI in the intestine.
p<>. In a study transgenic mice over-expressing SR-BI primarily in the intestine were generated. The transgene contains the mouse SR-BI gene under the control of the human intestinal specific apolipoprotein (apo) C-III enhancer coupled with the apo A-IV promoter.
This construct induces decreasing SR-BI expression along the gastrocolic axis and an increasing one from the crypt to the top of the villosity.
Thus, SR-BI is expressed where intestinal cholesterol absorption mainly occurs in SR-BI transgenic mice as in wild type littermate.
This study evidences for the first time that SR-BI plays a role in vivo in intestinal cholesterol absorption. Indeed, the intestinal cholesterol uptake determined by radioactive acute phase measurement of plasma cholesterol was doubled at 3 hours after gavage in the transgenic mice. In addition a net increase of tocopherol, a specific marker for chylomicron synthesis, was measured in the plasma of the transgenic mice following gavage with tocopherol.
Interestingly, in contrast to the increased intestinal cholesterol uptake, they observed an unexpected decrease of plasma cholesterol content in all lipoprotein fractions (around 50%), which resembles to the major phenotype observed in animals over-expressing SR-BI in the liver.
A possible explanation is that the rise of intestinal cholesterol absorption is likely balanced by the moderate over expression of SR-BI in the liver inducing an increase of cholesterol catabolism shown by the bile cholesterol secretion enhancement as well as an increase of chylomicrons catabolism.
Liver SR-BI overexpression causes a decrease in plasma apo AI levels increasing HDL clearance in liver, but in this transgenic model, a diminution in plasma apo AI was not observed, whereas HDL cholesterol was decreased by two fold. Apo A-I can be synthesized by the liver through HDL formation or by the intestine associated with chylomicron secretion. Maybe, the intestine could participate in the regulation of apo AI synthesis through an increase of chylomicron secretion, which do not modify cholesterol plasmatic levels, containing mostly triglycerides.
Accelerated lipid absorption in mice overexpressing intestinal SR-BI
HORMONAL REGULATORY MECHANISMS
Cholesterol and lipoprotein metabolism is regulated by some hormonal systems:
1.Thyroid hormones regulate cholesterol and lipoprotein metabolism(, but cardiac effects restrict their use as hypolipidemic drugs). T3 binds to thyroid hormone receptors TRsα and β. TRβ is the predominant isoform in liver, whereas T3 effects on heart rate are mediated mostly by TRα.
The large reduction of plasma HDL cholesterol induced by T3 is not accompanied in mice by increased intrahepatic cholesterol content, so that it can be hypotyzed that increased hepatic uptake of HDL-cholesterol via SR-BI, is paralleled by elimination of cholesterol as bile acids. In other words, the increased cholesterol excretion with feces takes place in an indirect way, through an increased excretion of bile acids derived from cholesterol.
Experimental drugs that target TRβ or exhibit tissue-selective uptake may reduce plasma lipid levels while sparing the heart, unlike T3, whose use is limited because of its cardiac effects due to its low selectivity.
Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice
2.Estrogens. In mice high doses of E2 augment intestinal cholesterol absorption, mostly due to an upregulated expression of intestinal sterol influx transporter NPC1L1 via the intestinal ER pathway and an increase in bile cholesterol secretion through the hepatic ER pathway
In addition, the ER activated by E2 promotes hepatic outputs of bile lipids, probably by increasing canalicular expression of ABCG5 and ABCG8.
Then, estrogens could stimulate HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis so that more total-body cholesterol is produced in the liver and small intestine. The increase in hepatic cholesterol biosynthesis is associated with a significant increase in secretion rates of bile total and newly synthesized cholesterol. Consequently, there is an increased delivery of cholesterol from bile into the intestinal lumen for absorption by the enterocyte.
Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: gender and age effects