A wart is generally a small, rough growth, typically on a human’s hands or feet but often other locations, that can resemble a cauliflower or a solid blister. They are caused by a viral infection, specifically by one of the many types of human papillomavirus (HPV). There are as many as 10 varieties of warts, the most common considered to be mostly harmless. It is possible to get warts from others; they are contagious and usually enter the body in an area of broken skin.They typically disappear after a few months but can last for years and can recur.


Types
A range of types of wart have been identified, varying in shape and site affected, as well as the type of human papillomavirus involved. These include:
- Common wart (Verruca vulgaris), a raised wart with roughened surface, most common on hands, but can grow anywhere on the body.
- Flat wart (Verruca plana), a small, smooth flattened wart, flesh-coloured, which can occur in large numbers; most common on the face, neck, hands, wrists and knees;
- Filiform or digitate wart, a thread- or finger-like wart, most common on the face, especially near the eyelids and lips.
- Genital wart (venereal wart, Condyloma acuminatum, Verruca acuminata), a wart that occurs on the genitalia.
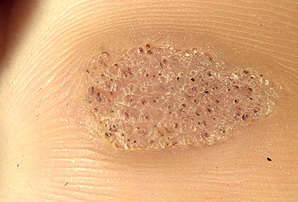
- Mosaic wart, a group of tightly clustered plantar-type warts, commonly on the hands or soles of the feet.
- Periungual wart, a cauliflower-like cluster of warts that occurs around the nails.
- Plantar wart (verruca, Verruca plantaris), a hard sometimes painful lump, often with multiple black specks in the center; usually only found on pressure points on the soles of the feet.
Cause
Warts are caused by the human papilloma virus (HPV). There are about 130 known types of human papilloma viruses. HPV infects the squamous epithelium, usually of the skin or genitals, but each HPV type is typically only able to infect a few specific areas on the body. Many HPV types can produce a benign growth, often called a "wart" or "papilloma", in the area they infect. Many of the more common HPV and wart types are listed below.
- Common warts: HPV types 2 and 4 (most common); also types 1, 3, 26, 29, and 57 and others.
- Cancers and genital dysplasia: "high-risk" HPV types are associated with cancers, notably cervical cancer, and can also cause some vulvar, vaginal, penile, anal and some oropharyngeal cancers. "low-risk" types are associated with warts or other conditions.
- High-risk: 16, 18 (cause the most cervical cancer); also 58, 33, 45, 31, 52, 35, 39, 59, and others.
- Plantar warts (myrmecia): HPV type 1 (most common); also types 2, 3, 4, 27, 28, and 58 and others.
- Anogenital warts (condylomata acuminata or venereal warts) - HPV types 6 and 11 (most common); also types 42, 44 and others.
- Low-risk: 6, 11 (most common); also 13, 44, 40, 43, 42, 54, 61, 72, 81, 89, and others.
- Flat warts: HPV types 3, 10, and 28.
- Butcher's warts: HPV type 7.
- Heck's disease (Focal epithelial hyperplasia): HPV types 13 and 32.
Pathophysiology
Warts can affect any area on the skin and mucous membranes. The HPV virus infects the epithelium, and systemic dissemination of the virus does not occur. Viral replication occurs in differentiated epithelial cells in the upper level of the epidermis; however, viral particles can be found in the basal layer. Common warts have a characteristic appearance under the microscope. They have thickening of the stratum corneum (hyperkeratosis), thickening of the stratum spinosum (acanthosis), thickening of the stratum granulosum, rete ridge elongation, and large blood vessels at the dermoepidermal junction.
Prevention
Gardasil is an HPV vaccine aimed at preventing cervical cancers and genital warts. Gardasil is designed to prevent infection with HPV types 16, 18, 6, and 11. HPV types 16 and 18 currently cause about 70% of cervical cancer cases, and also cause some vulvar, vaginal, penile and anal cancers. HPV types 6 and 11 are responsible for 90% of documented cases of genital warts. Unfortunately the HPV vaccines do not currently prevent the virus strain responsible for verrucas (plantar warts).
Treatment
- Keratolysis, of dead surface skin cells usually using salicylic acid, blistering agents, immune system modifiers ("immunomodulators"), or formaldehyde, often with mechanical paring of the wart with a pumice stone, blade etc.
- Electrodesiccation.
- Cryosurgery, which involves freezing the wart (generally with liquid nitrogen), creating a blister between the wart and epidermal layer, after which the wart and surrounding dead skin falls off by itself. An average of 3 to 4 treatments are required for warts on thin skin. Warts on calloused skin like plantar warts might take dozens or more treatments.
- Surgical curettage of the wart;
- Laser treatment often with a pulse dye laser or carbon dioxide (CO2) laser. Pulse dye lasers (wavelength 582 nm) work by selective absorption by blood cells (specifically haemoglobin). CO2 lasers work by selective absorption by water molecules. Pulse dye lasers are less destructive and more likely to heal without scarring. CO2 laser works by vaporizing and destroying tissue and skin. Laser treatments can be painful, expensive (though covered by many insurances), and can cause little scarring when used appropriately. CO2 lasers will require local anaesthetic. Pulse dye laser treatment does not need conscious sedation nor local anesthetic. It takes 2 to 4 treatments but can be many more for extreme cases. Typically, 10-14 days are required between treatments. Preventative measures are important.
- Infrared coagulator an intense source of infrared light in a small beam like a laser. This works essentially on the same principle as laser treatment. It is less expensive. Like the laser, it can cause blistering pain and scarring.
- Duct tape occlusion therapy involves placing a piece of duct tape over the wart. The evidence as to whether or not it is effective is poor. Thus it is not recommended as routine treatment.
Cryotherapy

Cryotherapy is a technique that uses an extremely cold liquid or instrument to freeze and destroy abnormal skin cells that require removal. The technique has been in use since the turn of the century, but modern techniques have made it widely available to dermatologists and primary care doctors. The technique is also known as cryocautery or cryosurgery. In dermatology applications, there are three main techniques used in cryotherapy. In the simplest technique, usually reserved for warts and other benign skin growths, the physician dips a cotton swab or other applicator into a cup containing a "cryogen" such as liquid nitrogen and applies it directly to the skin growth to freeze it. At a temperature of –320°F (–196°C), liquid nitrogen is the coldest cryogen available. The goal is to freeze the skin growth as quickly as possible, and then let it thaw slowly to cause maximum destruction of the skin cells. A second application may be necessary depending on the size of the growth. In another approach, a device is used to direct a small spray of liquid nitrogen or other cryogen directly onto the skin growth. Freezing may last from five to 20 seconds, depending on the size of the lesion. A second freeze-thaw cycle may be required. Sometimes, the physician inserts a small needle connected to a thermometer into the lesion to make certain the lesion is cooled to a temperature low enough to guarantee maximum destruction. In a third option, liquid nitrogen or another cryogen is circulated through a probe to cool it to low temperatures. The probe is then brought into direct contact with the skin lesion to freeze it. The freeze time can take two to three times longer than with the spray technique.
Preparation: No extensive preparation is required prior to cryotherapy. The area to be treated should be clean and dry, but sterile preparation is not necessary. Patients should know that they will experience some pain at the time of the freezing, but local anesthesia is usually not required. In dermatology applications, the physician may want to reduce the size of certain growths such as warts prior to the cryotherapy procedure, and may have patients apply salicylic acid preparations to the growth over several weeks. Sometimes, the physician will pare away some of the tissue using a device called a curette or a scalpel. In the case of cervical cryotherapy , the procedure is not performed during, or from two to three days before, the menstrual period.
Aftercare: In dermatology applications, redness, swelling, and the formation of a blister at the site of cryotherapy are all expected results of the treatment. A gauze dressing is applied, and patients should wash the site three or four times daily while fluid continues to ooze from the wound, usually for five to 14 days. A dry crust will form that falls off by itself. Wounds on the head and neck may take four to six weeks to heal, but those on the body, arms, and legs can take longer. Some patients experience pain at the site following the treatment. This can usually be eased with acetaminophen, though in some cases a stronger pain reliever may be required.
Risks: In dermatology applications, cryotherapy poses little risk and can be well tolerated by elderly and other patients who are not good candidates for other surgical procedures. As with other surgical procedures, there is some risk of scarring, infection, and damage to underlying skin and tissue. These risks are generally minimal in the hands of experienced physicians. Care should be taken, however, in subjecting people with diabetes or certain circulation problems to cryotherapy for growths located on their lower legs, ankles, and feet. In these patients, healing can be poor and the risk of infection can be higher than for other patients. Although cryotherapy is a relatively low-risk procedure, some side effects may occur as a result of the treatment. They include:
Infection. Though uncommon, infection is more likely on the lower legs where healing can take several months.
Pigmentary changes. Both hypopigmentation (lightening of the skin) and hyperpigmentation (darkening of the skin) are possible after cryotherapy. Both generally last a few months, but can be longer lasting.
Nerve damage. Though rare, damage to nerves is possible, particularly in areas where they lie closer to the surface of the skin, such as the fingers, the wrist, and the area behind the ear. Reports suggest this will disappear within several months.
Normal results: Some redness, swelling, blistering, and oozing of fluid are all common results of cryotherapy. Healing time can vary by the site treated and the cryotherapy technique used. When cryogen is applied directly to the growth, healing may occur in three weeks. Growths treated on the head and neck with the spray technique may take four to six weeks to heal, while growths treated on other areas of the body may take considerably longer. Cryotherapy boasts high success rates in permanently removing skin growths; even for malignant lesions such as squamous cell and basal cell cancers, studies have shown a cure rate of up to 98%. For certain types of growths such as some forms of warts, repeat treatments over several weeks are necessary to prevent the growth's return.
Salicylic acid

Salicylic acid is a monohydroxybenzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prodrug to salicylic acid, it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.
Chemistry: Salicylic acid has the formula C6H4COOH, where the OH group is ortho to the carboxyl group. It is also known as 2-hydroxybenzoic acid. It is poorly soluble in water (2 g/L at 20 °C). Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.
Plant hormone: Salicylic acid (SA) is a phenolic phytohormone and is found in plants with roles in plant growth and development, photosynthesis, transpiration, ion uptake and transport. SA also induces specific changes in leaf anatomy and chloroplast structure. SA is involved in endogenous signaling, mediating in plant defense against pathogens. It plays a role in the resistance to pathogens by inducing the production of pathogenesis-related proteins. It is involved in the systemic acquired resistance (SAR) in which a pathogenic attack on one part of the plant induces resistance in other parts. The signal can also move to nearby plants by salicylic acid being converted to the volatile ester, methyl salicylate.
Production: Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can also be synthesized via a phenylalanine-independent pathway. Sodium salicylate is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid: It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of wintergreen) with a strong acid or base.
Medicinal and cosmetic uses: Salicylic acid is known for its ability to ease aches and pains and reduce fevers. These medicinal properties, particularly fever relief, have been known since ancient times, and it is used as an anti-inflammatory drug. Cotton pads soaked in salicylic acid can be used to chemically exfoliate skin. As with other beta hydroxy acids, salicylic acid is a key ingredient in many skin-care products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, corns, keratosis pilaris, and warts. It works as a keratolytic, bacteriocide and comedolytic agent by causing the cells of the epidermis to shed more readily, opening clogged pores and neutralizing bacteria within, preventing pores from clogging up again by constricting pore diameter, and allowing room for new cell growth. Use of concentrated solutions of salicylic acid may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock.
Mechanism of action: Salicylic acid has been shown to work through several different pathways. It produces its anti-inflammatory effects via suppressing the activity of cyclooxygenase (COX), an enzyme which is responsible for the production of pro-inflammatory mediators such as the prostaglandins. Notably, it does this not by direct inhibition of COX, unlike most other non-steroidal anti-inflammatory drugs (NSAIDs), but instead by suppression of the expression of the enzyme (via a yet-unelucidated mechanism). Salicylic acid has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK), and it is thought that this action may play a role in the anticancer effects of the compound and its prodrugs aspirin and salsalate. In addition, the antidiabetic effects of salicylic acid are likely mediated by AMPK activation primarily through allosteric conformational change that increases levels of phosphorylation. Salicylic acid also uncouples oxidative phosphorylation which leads to increased ADP:ATP and AMP:ATP ratios in the cell. Consequently, salicylic acid may alter AMPK activity and subsequently exert its anti-diabetic properties through altered energy status of the cell. Even in AMPK knock-out mice, however, there is an anti-diabetic effect demonstrating that there is at least one additional, yet-unidentified action of the compound. Salicylic acid is a key ingredient in many skin-care products for the treatment of acne, psoriasis, calluses, corns, keratosis pilaris, and warts. It works by causing the cells of the epidermis to slough off more readily, preventing pores from clogging up, and allowing room for new cell growth. Salicylic acid is also used as an active ingredient in gels which remove verrucas (plantar warts). Salicylic acid inhibits the oxidation of uridine-5-diphosphoglucose (UDPG) competitively with nicotinamide adenosine dinucleotide (NAD) and noncompetitively with UDPG. It also competitively inhibits the transferring of glucuronyl group of uridine-5-phosphoglucuronic acid (UDPGA) to the phenolic acceptor. The wound-healing retardation action of salicylates is probably due mainly to its inhibitory action on mucopolysaccharide synthesis.
Safety: Topically, as a beta-hydroxy acid (and unlike alpha-hydroxy acids) salicylic acid is capable of penetrating and breaking-down fats and lipids, making it capable of causing moderate chemical burns of the skin if at very high concentrations. It is capable of damaging the lining of pores in such cases if the solvent is alcohol, acetone, or an oil. Over-the-counter limits are set at 2% for topical left on the face and 3% for those expected to be washed off, such as acne cleansers or shampoo. Caution should be exercised when handling large volumes of salicylic acid, and protective gloves are recommended for any repeat, prolonged exposure. 17% salicylic acid, which is often sold for wart removal, should not be applied to the face and should not be used for acne treatment. Even for wart removal, such a solution should only be applied twice a day – more frequent use may lead to an increase in side effects without an increase in efficacy.
Salicylic acid or cryotherapy?
PUBMED: http://www.ncbi.nlm.nih.gov/pubmed/20837684
METHODS: Consecutive patients with new cutaneous warts were recruited in 30 primary care practices in the Netherlands between May 1, 2006, and Jan. 26, 2007. We randomly allocated eligible patients to one of three groups: cryotherapy with liquid nitrogen every two weeks, self-application of salicylic acid daily or a wait-and-see approach. The primary outcome was the proportion of participants whose warts were all cured at 13 weeks. Analysis was on an intention-to-treat basis. Secondary outcomes included treatment adherence, side effects and treatment satisfaction. Research nurses assessed outcomes during home visits at 4, 13 and 26 weeks.
RESULTS: Of the 250 participants (age 4 to 79 years), 240 were included in the analysis at 13 weeks (loss to follow-up 4%). Cure rates were 39% (95% confidence interval [CI] 29%-51%) in the cryotherapy group, 24% (95% CI 16%-35%) in the salicylic acid group and 16% (95% CI 9.5%-25%) in the wait-and-see group. Differences in effectiveness were most pronounced among participants with common warts (n = 116): cure rates were 49% (95% CI 34%-64%) in the cryotherapy group, 15% (95% CI 7%-30%) in the salicylic acid group and 8% (95% CI 3%-21%) in the wait-and-see group. Cure rates among the participants with plantar warts (n = 124) did not differ significantly between treatment groups.
INTERPRETATION: For common warts, cryotherapy was the most effective therapy in primary care. For plantar warts, we found no clinically relevant difference in effectiveness between cryotherapy, topical application of salicylic acid or a wait-and-see approach after 13 weeks.
PUBMED: http://www.ncbi.nlm.nih.gov/pubmed/21899812
OBJECTIVE: To compare the clinical effectiveness and cost-effectiveness of cryotherapy using liquid nitrogen versus patient daily self-treatment with 50% salicylic acid for the treatment of verrucae (plantar warts).
DESIGN: A multicentre, pragmatic, open, two-armed randomised controlled trial with an economic evaluation. Randomisation was simple, with the allocation sequence generated by a computer in a 1 : 1 ratio.
SETTING:Podiatry clinics, university podiatry schools and primary care in England, Scotland and Ireland.
PARTICIPANTS: Patients were eligible if they presented with a verruca which, in the opinion of the health-care professional, was suitable for treatment with both salicylic acid and cryotherapy, and were aged 12 years and over.
INTERVENTIONS: Cryotherapy using liquid nitrogen delivered by a health-care professional compared with daily patient self-treatment with 50% salicylic acid (Verrugon, William Ransom & Son Plc, Hitchin, UK) for a maximum of 8 weeks.
MAIN OUTCOME MEASURES: The primary outcome was complete clearance of all verrucae at 12 weeks. Secondary outcomes were complete clearance of all verrucae at 12 weeks, controlling for age, whether or not the verrucae had been previously treated and type of verrucae, with a second model to explore the effect of patient preferences, time to clearance of verrucae, clearance of verrucae at 6 months, number of verrucae at 12 weeks and patient satisfaction with the treatment.
RESULTS: In total, 240 eligible patients were recruited, with 117 patients allocated to the cryotherapy group and 123 to the salicylic acid group. There was no evidence of a difference in clearance rates between the treatment groups in the primary outcome [17/119 (14.3%) in the salicylic acid group vs 15/110 (13.6%) in the cryotherapy group; p = 0.89]. The results of the study did not change when controlled for age, whether or not the verrucae had been previously treated and type of verrucae, or when patient preferences were explored. There was no evidence of a difference in time to clearance of verrucae between the two groups [hazard ratio (HR) 0.80, 95% confidence interval (CI) 0.51 to 1.25; p = 0.33] or in the clearance of verrucae at 6 months (33.7% cryotherapy vs 30.5% salicylic acid). There was no evidence of a difference in the number of verrucae at 12 weeks between the two groups (incidence rate ratio 1.08, 95% CI 0.81 to 1.43; p = 0.62). Nineteen participants reported 28 adverse events, 14 in each group, with two treatment-related non-serious adverse events in the cryotherapy group. Cryotherapy was also associated with higher mean costs per additional healed patient (£101.17, 95% bias-corrected and accelerated CI £85.09 to £117.26). The probability of cryotherapy being cost-effective is 40% for a range of willingness-to-pay thresholds of £15,000-30,000 per patient healed.
CONCLUSIONS: There is no evidence for a difference in terms of clearance of verrucae between cryotherapy and salicylic acid (at both 12 weeks and 6 months), number of verrucae at 12 weeks and time to clearance of verrucae. Cryotherapy was associated with higher mean costs per additional healed patient compared with salicylic acid.
Wikipedia
Surgeryencyclopedia
Pubmed
Medical-dictionary
Pubmed