DEFINITION
Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver. Albumin constitutes about half of the blood serum protein. It is soluble and monomeric.
Human serum albumin (HSA):
- (i) supply specific aminoacids to peripheral tissues
(ii) controls the plasma oncotic pressure and modulates fluid distribution between the body compartments,
(iii) represents the depot and carrier of endogenous and exogenous compounds,
(iv) increases the apparent solubility and lifetime of hydrophobic compounds,
(v) affects pharmacokinetics of many drugs,
(vi) inactivates toxic compounds,
(vii) induces chemical modifications of some ligands,
(viii) displays antioxidant properties, and
(ix) shows enzymatic properties.
(x) binds Ca++ ions
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure

Protein Aminoacids Percentage (Width 700 px)


SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
BIOLOGICAL FUNCTIONS
Bilirubin transport
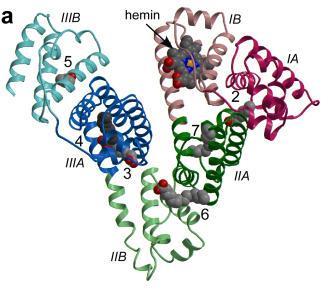
HSA, the primary transport vehicle for bilirubin species in blood, is a highly soluble protein present in plasma at a concentration of around 0.6 mM in healthy adults. The monomeric protein is composed of three structurally homologous helical domains (I, II and III), each of which is divided into two sub-domains (A and B).HSA preferentially binds lipophilic anions and serves as a transporter for a variety of small-molecule ligands. Its primary cargo appears to be non-esterified fatty acids but the protein is also known to have a high affinity for other endogenous anions, such as bilirubin, heme and thyroxine.
The 4Z,15Z-isomer of bilirubin binds with high affinity (KD ~ 16 nM) to a single site on HSA.There are also one or two secondary sites with affinities that are at least tenfold lower.These are thought to be unimportant in vivobut can complicate the interpretation of in vitro binding experiments.Despite numerous studies, the location of the primary bilirubin-binding site on albumin is not known. There is evidence that it is not in drug site 2, within sub-domain IIIA,but evidence in favor of either the heme-binding site in sub-domain IB or the drug-binding site 1 in subdomain IIA is equivocal and contradictory. Investigations using compounds that bind specifically to drug site 1 in sub-domain IIA have produced results that are non-trivial to interpret at the molecular level.
| Binding site | Binding competitors |
| Site1/Subdomain IIA | Bilirubin, warfarin, phenylbutazone, azapropazone, indomethacin, iophenoxic acid, thyroxine |
However, in some cases displacement of bilirubin was observed only at high concentrations of competitor which raises the possibility that displacement of bilirubin may be due to drug binding at secondary sites outside sub-domain IIA.
Crystallographic analysis reveals the structural basis of the high-affinity binding of iophenoxic acid to human serum albumin, 2011
Hematin binding
Reconstitution of Panhematin™ with Human Albumin: Instructions for Preparation and Intravenous Infusion
HSA takes out heme from high- and low-density lipoproteins and transfers the metal-macrocycle to hemopexin. After endocytosis of the hemopexin-heme complex into the hepatic parenchymal cells through the CD91 receptor, hemopexin releases heme intracellularly.

Three-dimensional structure of HSA complexed with endogenous and exogenous ligands bound to the FA sites. The subdomains of HSA are rendered with different colors (domain IA, in blue; domain IB, in cyan; domain IIA, in forest green; domain IIA, in green; domain IIIA, in yellow; domain IIIB, in orange). The FA1-salicylic acid (PDB ID: 2I30),36 F2-capric acid (PDB ID: 1E7E),17 FA3-FA4-ibuprofen (PDB ID: 2BXG),15 FA5-propofol (PDB ID: 1E7A),35 FA6-halothane (PDB ID: 1E7C),35 FA7-warfarin (PDB ID: 2BXD),15 F8-capric acid (PDB ID: 1E7E),17 and FA9-thyroxine (PDB ID: 1HK4)39 complexes are highlighted. Three molecules of halothane are bound in the FA6 site
The FA1 binding site (located in subdomain IB) binds very different ligands (e.g., heme, bilirubin, FAs, and exogenous compounds) and has been reported to represent a major drug-binding pocket.
Catalytic actions:
- The HSA-heme-Fe(II)-catalyzed conversion of NO2− to NO
- O2-based scavenging of NO-bound HSA-heme-Fe(II)
- Peroxynitrite detoxification by HSA-heme-Fe(III)
- Catalase and peroxidase activity of HSA-heme-Fe(III)
Paolo Ascenzi, Heme-based catalytic properties of human serum albumin, 2015
- Under physiological and pathological conditions, HSA has a pivotal role in heme scavenging transferring the metal-macrocycle from high- and low-density lipoproteins to hemopexin, thus acquiring globin-like reactivity. Here, the heme-based catalytic properties of HSA are reviewed and the structural bases of drug-dependent allosteric regulation are highlighted.
REGULATION
DIAGNOSTIC USE